Basic Neurospora protocols from the lab of D.
E. A. Catcheside,
NEUROSPORA METHODS
This manual documents
common methods used for the culture of Neurospora in the Catcheside Laboratory
at
CONTENTS
TITLE SECTION
PRECAUTIONS 3
GENERAL CULTURE METHODS
Tube cultures 3
Liquid cultures 3
Storage of cultures 4
Growth tests to
determine genotype 4
Quantitative growth
tests 5
Mating type tests 5
Growth restriction
techniques 6
Crossing methods 6
Analysis of crosses Random spore
analysis I 6
Random
spore analysis II 7
Non-random
spores 8
INOCULATION METHODS
Conidial Suspensions I. Small scale for subculturing 8
II. Medium scale for inoculation of multiple
tests or
inoculation of liquid medium 9
III. Large scale conidial suspensions for
preparation
of spheroplasts etc. 9
Mycelial fragments 9
ISOLATION OF MUTANTS 10
RECOMBINATION ASSAYS 11
MEDIA
Vegetative growth: 1. Liquid Medium 12
2. Slopes 13
3. Plates 13
4. Layer Agar 14
Crossing medium: 1. Tubes 14
2. Plates and Tubes
STOCK SOLUTIONS:
Vogel's X 50 15
Trace elements 15
SS X 20 16
SS (nit-2) x 20 16
SGF X 20 16
Westergaard's X 4 16
SC X 2 17
AWM X 4 17
SUPPLEMENTS 18
CONVENTIONS USED FOR LABELLING MEDIUM:
|
MEDIUM TYPE |
TYPICAL USES |
LABEL AS |
|
Sucrose as C source |
tube medium for stock
cultures and tests, liquid medium for flasks |
VM +
supplements (if any) |
|
Sorbose with glucose
and fructose as carbon sources |
plating medium |
SGF + supplements (if any) |
|
Sorbose with sucrose
as carbon source |
plating medium (less
growth restriction) |
SS + supplements (if any) |
|
Top layer agar with 1
M sorbitol |
regeneration of
spheroplasts |
HARD TOP + supplements (if any) |
|
Top layer agar |
plating of ascospores
or conidia |
TOP + supplements (if any) |
PRECAUTIONS
Neurospora has a
reputation of being a troublesome air-borne laboratory contaminant. This is
because the conidia are readily detached from their conidiophores. Although there is no health risk, your
experiments and those of others working with slower growing organisms could
suffer from careless handling of Neurospora cultures. Careful technique and a little thought will
effectively prevent this potential problem and essentially all fungal
contaminants will turn out to be those ubiquitously present in the air entering
the laboratory; usually Penicillium or Aspergillus. If you are unfortunate enough to create a
cloud of conidia, cover all equipment then spray the laboratory air with a
mixture composed of equal volumes of ethanol and 10% aqueous propylene glycol
and retire from the room. Having allowed
time for the aerosol to settle, wipe all horizontal surfaces. The spray will trap and settle airborne dust
and spores but will create a sticky deposit on exposed surfaces. This will
evaporate eventually but may damage absorbent materials that are not covered.
Tube cultures
Neurospora is
conveniently cultured on 1.5 ml agar slopes of Vogel's N minimal medium
(supplemented with specific growth factors as required) in 100 x 13 mm tubes
plugged with non-absorbent cotton wool.
Such cultures are sufficiently small to handle in large numbers but
provide sufficient conidia for initiating most types of experiment. Growth is most rapid at 34o but
stocks containing the colonial temperature sensitive mutation cot-1 must
be grown at 25o to obtain conidia.
Conidiation can be improved by moving the tubes into diffuse light at
room temperature after conidiation has started (i.e. after 18-24 hr. at 34o,
40-48 hr. at 25o).
Large numbers of
conidia can be produced from a 100 ml layer of agar-solidified Vogel's medium
in a 1 l conical flask.
Liquid cultures
Submerged cultures of
laboratory strains do not conidiate.
Liquid Vogel's N medium is used.
The yield of mycelium is about 30 g fresh weight/l.
Aeration may be
achieved by shaking; use flasks plugged with cotton or closed with loose
fitting metal caps, containing 1/5 the flask volume of medium, and adjust the
shaker to give vigorous mixing without splashing the plug. With care, up to half a flask volume of
medium can be used; particularly on orbital shakers but under aeration is a
common problem when this is tried and poor growth is likely. At 25o growth is complete in about
72 hours.
Larger vessels may be
aerated by sparging with compressed air sterilised by passage through a tube
loosely packed with non absorbent cotton wool.
The glass sparge tube should be inserted deep into the medium and the
air flow adjusted to give good vertical mixing.
8 l of medium in a 10 l capacity bottle is satisfactory.
Storage of cultures
Temporary
Cultures grown on
slopes, suitably supplemented, can be kept at 5o following
incubation and will normally remain viable for several months. At -20o, the tubes remain viable
for several years.
Long term
The most convenient
method for long term storage is on silica-gel.
Half fill 100 x 13 mm tubes with 12-20 mesh indicator free silica-gel
granules, plug with rolled muslin, cover plugs with foil and sterilise at 160oC
for one and a half hours in an oven.
Store sterile tubes over anhydrous calcium chloride in a
desiccator. Dispense 1.5 ml of
reconstituted dried skim-milk per 100 m x 13 mm tube, cap and autoclave 5 min.
at 121oC. Store the tubes at
room temperature and on each of next two days steam the tubes for half an
hour. Prepare 1.5 ml tubes of sterile
water and use these to add 1.5 ml of sterile water to a 3-7 day old conidiating
culture, suspend conidia and then add 1.5 ml sterile skim milk. Mix and allow the conidia to settle out ( 0.5
hr). Don't be tempted to try and suspend
the conidia directly in skim milk, it is very difficult. When the conidia are
settled, use a pasteur pipette to remove about half of the supernatant and
resuspend the conidia in the remainder.
Before opening, hold each gel tube horizontal and shake the gel to
provide an air gap to the base of the tube.
Then using a pasteur pipette, distribute about 0.8 ml of suspension
evenly over the granules in each of the two silica gel tubes. Shake the gel periodically until all of
liquid is absorbed then dry the tubes in
vacuo over CaCl2 for 2-3 days.
Cover the tubes with parafilm and store dry at 5o. Preparations made in this way remain viable
for many years. Several hundred
subcultures may be made from each tube.
To subculture, tip a few silica gel granules onto a slope of appropriate
medium and incubate. Reseal the gel tube
and return to 5o.
Aconidiate strains can
be preserved on silica gel. Grow in
tubes of liquid medium containing sterile glass beads. Vortex mix after growth and add the resulting
broken mycelium to skim milk. Treat as
for conidia.
Growth tests to
determine genotype
If a culture contains
an auxotrophic mutation it will not grow properly, if at all, in the absence of
its specific growth requirement. Other
mutations confer resistance to antimetabolites and enable growth on media toxic
to the wild-type. Tests for ability to
grow on Vogel's minimal medium supplemented with different combinations of
growth factors enable determination of the genotype of multiple mutants. A control medium on which all mutant
combinations can grow should always be used.
Incubation at different temperatures enables the identification of
temperature sensitive mutants. Tests may
be performed in tubes or on plates ruled into a grid of 16, 25 or any other
convenient number of squares. Test media
may be inoculated with conidia or small pieces of mycelium cut from a colony
growing on an agar plate. When plates
are used, a technique to restrict growth is essential (q.v.).
Quantitative growth
tests
Use flasks of liquid
medium supplemented as required. Harvest
over a buchner after growth is complete (or earlier if plotting a growth
curve); wash with distilled water, wrap in weighed foil and dry at 60-80oC
to constant weight. Flasks can be
incubated without shaking but more reliable results are obtained by aerating
the flasks by shaking them on a reciprocating or orbital shaker. For reciprocating shaker, fill the flasks with
1/5 volume of medium. For orbital
shakers, somewhat larger volumes can be used.
Shaking speeds should be adjusted to give vigorous aeration without
splashing the plugs; the precise speed will depend upon the geometry of the
flask. Cotton plugs should not be too
tightly rolled and metal thimble caps are a good substitute since they permit
good gas exchange. Flasks with uneven
neck size or which are unevenly plugged, may give variable growth yields.
Mating type tests
(i) By
using standard crossing methods (q.v.) and pairing the unknown with each of two
standard strains, one A, the other a mating type,
|
1 |
(ii) Inoculate
the centre of a petri dish of Westergaard or SC agar medium with the fluffy a mating type tester and a second
dish with a fluffy A tester. The fluffy
mutant is aconidiate and thus safe to grow in petri dishes. Normal strains will grow out of the plate,
conidiate, and cause contamination problems. Incubate for 5 days at 25o,
check for the present of protoperithecia, rule mycelial mats into 16-25 squares
with a sterile needle and test unknowns by inoculating one square on each
mating type tester with a dab or conidia.
A single loopful of conidia can be used for both plates since there is
little danger of transferring the aconidiate tester from one plate to the
other. Incubate at 25o and
score for perithecial development at 18-48 hr.
(iii) Inoculate
1.5 ml slopes of SC medium in 100 x 13 mm tubes with fluffy testers. Incubate 5
days at 25o, check for perithecia and store at 4o for up
to 4-6 weeks before use. Inoculate with
unknown, incubate at 25o for 24-48 hr.
Growth restriction
techniques
Sorbose is not utilised
by Neurospora, it causes rupture of hyphal tips and stimulates branching
growth. Two sugar mixtures are commonly
used in place of sucrose, SGF being the most restrictive.
SGF: 1% sorbose, 0.05% glucose, 0.05% fructose
SS: 1% sorbose, 0.1% sucrose.
These are made as 20 x
stock solutions, sterilised by autoclaving, stored at 4o and added
aseptically to pre-sterilised medium.
Colonies will be normally be visible after 24 hours and well grown by 48
- 72 hours. Plates should be disposed of
or transferred to 4o after this time or conidiation may occur.
The colonial
temperature sensitive mutant cot-1
C102t gives tightly restricted dense growth at 34o. Incubation at this temperature immediately
following plating may prevent "visible" colonies developing for some
days, however, very large numbers of colonies may be screened on each plate. It is convenient to incubate cot-1 conidia or ascospores for 18-24 hr
at 25o on SS or SGF medium then transfer to 34o for 24-48
hr. This yields star like colonies which
are easily visible to the unaided eye.
Crossing methods
Crosses are
conveniently performed in 150 x 16 mm cotton plugged test tubes containing 4 ml
of liquid Westergaard's medium supplemented as necessary and a 6 cm x 4 cm
spill of Whatman #1 filter paper folded lengthwise into a "W". Normally conidia from both parents are added
together, mixed and spread evenly over the paper by inclining and rotating the
tube. If it is desirable to have only
one parent acting as female, inoculate the female parent first, incubate
for 5 days or until protoperithecia are
present, then wet the culture surface with a conidial suspension prepared from
the male parent; decant excess suspension.
Crosses should be incubated at 25o. Ascospores are normally produced in 5-20 days
depending upon the cross. If genetic
analysis is intended, wait 7 days after
the first spores are discharged from the perithecia to ensure full maturation
of the different genotypes. Maturation
of spores can be speeded by incubation at 30o following their
discharge but this will prevent further meiosis.
ANALYSIS OF CROSSES
Ascospores must be heat
treated to induce germination. 1 hour in
a 60o water bath is more than adequate and serves to kill
conidia. The heat treatment should not
be interrupted as ascospores will start to germinate within minutes and are
killed by further heating. The heat
treatment can usually be shortened to 30 minutes or prolonged up to 2-3 hours
without serious loss of ascospore viability.
It is usually convenient to heat shock spores in suspension prior to
layering onto plates. If spores are
plated prior to the heat shock, use a 60o incubator and allow
additional time for the plates to warm-up.
Random spore analysis I
With a loop of sterile
water, transfer about 50-100 ascospores into a drop of sterile water on a
suitably supplemented plate of SGF or SS medium and spread the spores with
brisk strokes: rotate the plate at the same time to obtain an even
distribution.

To make a spreader,
seal the end of a pasteur pipette in a bunsen flame and bend it into shape or
make one from glass rod. Sterilise by
dipping in alcohol and flaming, but don't hold the spreader in the flame.
More efficient
spreading can be achieved by making a spore suspension in soft agar and
layering 3 ml on each plate.
Incubate the plate for
60 min at 60o and transfer to 25o for 24 hours or until
colonies are visible. After checking
under the dissecting microscope that the colony is derived from the growth of a
single ascospore, pick a small portion onto a slope of suitable medium, grow to
conidial stage and test genotype (q.v.).
Alternatively, pick small portions of each colony onto plates of test
medium.
This method has two
disadvantages: the proportion of viable ascospores is not determined and truly
random isolation of the colonies produced by germinated spores is
difficult. There may be an unconscious
operator effect preferentially picking colonies having a particular appearance;
an appearance often dictated by genotype.
Hence genetic ratios determined by this method may be biased.
Random spore analysis II
|
2 |
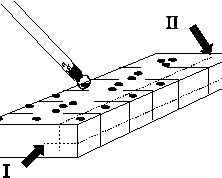
 Cut a 1½ cm x 3 cm block of agar from a plate and divide
each of the longer edges with 3 mm cuts (razor blade or microspatula) into
about 25 "keys".
Streak a loopful of spores down the centre of the block then using a loop
made from fine human hair (double a strand of hair into a capillary drawn on the
end of a pasteur pipette and fix it in place with epoxy glue), pull one black spore onto each key. Take care not to
lose spores in the cuts or mixed cultures will result. Colourless spores
will be inviable, make note if their frequency exceeds the normal 1-2%. Do not attempt to
sterilise the loop and work under a comfortable magnification (around 25x). When all keys have
a spore, cut under each key (I) and behind each spore (II). Using a spade ended
needle, transfer each block to a slope of suitably supplemented medium. Take care that the
spore is not lost during the transfer. Heat treat slopes at 60o in a water bath for one hour and incubate at 25o until conidia are produced then carry out tests to
determine the genotype.
Cut a 1½ cm x 3 cm block of agar from a plate and divide
each of the longer edges with 3 mm cuts (razor blade or microspatula) into
about 25 "keys".
Streak a loopful of spores down the centre of the block then using a loop
made from fine human hair (double a strand of hair into a capillary drawn on the
end of a pasteur pipette and fix it in place with epoxy glue), pull one black spore onto each key. Take care not to
lose spores in the cuts or mixed cultures will result. Colourless spores
will be inviable, make note if their frequency exceeds the normal 1-2%. Do not attempt to
sterilise the loop and work under a comfortable magnification (around 25x). When all keys have
a spore, cut under each key (I) and behind each spore (II). Using a spade ended
needle, transfer each block to a slope of suitably supplemented medium. Take care that the
spore is not lost during the transfer. Heat treat slopes at 60o in a water bath for one hour and incubate at 25o until conidia are produced then carry out tests to
determine the genotype.
With practice this method is as fast as the first, all
isolation manipulations are performed on one day instead of two and the number
of tubes in which no growth occurs measures the proportion of inviable
spores. Such
inviability may be associated with particular genes or gene combinations. A variation on this
method is to spread ascospores onto an agar plate then pick black spores singly
into tubes using a spade ended needle.
Non-random spores
It may be desirable to select particular genotypes in which
case method I can be employed and selective medium used for plating the spores.
Rare genotypes can often be obtained with relative ease by germinating spores
top layered onto a selective medium, marking all those that grow and then
overlaying the plate with a second top layer that contains an additional
nutrient and incubating again. Given an appropriate choice of medium, the
colonies that grow after the second incubation will be enriched for the genotype
you need.
INOCULATION METHODS
Always work near a flame and in a draft free area. Mop the bench with
a disinfectant prior to starting work. (Absolute alcohol is suitable but beware of
flames). When
handling cultures it is desirable to flame the neck of the vessel immediately on
opening and again immediately before reclosing. This may be impracticable with certain large
scale cultures and with petri dishes but is imperative with stock solutions
where contamination poses serious problems. When opening cultures, keep hold of the plugs
or caps, don't place them on the bench.
CONIDIAL SUSPENSIONS
Take care not to disperse conidia into the air. The yield of
conidia from a culture can usually be improved by removing from the incubator
into normal room light about 24-48 hours after conidiation commences. The viability of
conidia will start to decrease after 7-10 days storage at room temperature. This period can be
prolonged by storage at 4o.
I.
Small scale for making subcultures.
|
3 |
Flame a wire loop. When doing this, hold the loop nearly
vertical in the flame to get whole length of wire red hot. Cool the wire in a
petri-dish of sterile water and pick up a generous loopful of water. Open the culture,
flame neck of tube and holding tube and loop horizontal, touch the loop on the
wall of the tube near to the conidia to deposit a droplet, touch the loop
lightly onto the conidial mass, then puddle in the droplet to suspend
conidia. Pick
up a loopful of the suspension, tap wire lightly onto the wall of the tube to
remove any dry conidia, withdraw the loop from the tube and hold it stationary
whilst reflaming neck of culture tube and replacing the plug. The loop may now be
used for inoculating one to several tubes or marked squares on plates. Flame the neck of
the subculture tube on opening and reclosing. It is possible to hold both the culture and
subculture tube at the same time in one hand and manipulate both plugs and the
inoculating loop with the other hand.
With care, a single loop of conidial suspension can be used
to place conidial dabs in 2-3 tubes or plates thus saving labour when testing
nutritional mutants and mating types.
II. Medium scale for inoculation of multiple tests or
inoculation of liquid media.
Open the culture and a tube containing 1½ ml of sterile
water, hold the necks of the tubes inside a "cold" (luminous) bunsen flame and
tip into the culture.
This should be done carefully but quickly to avoid overheating the
water. Hold
tube necks inside flame for a few seconds to cremate any escaping conidia that
have been displaced by the addition of water to the tube and return the plug to
the culture tube as it is withdrawn from the
flame.
Leave the tube for a short time to avoid scorched fingers, then vortex
mix to produce a conidial suspension. Leave tube again for a short time to allow
any airborne conidia in the tube to settle and then remix.
To inoculate large numbers of differential test media, use
a sterile pasteur pipette as a capillary (no rubber teat) and touch onto the
appropriate squares on test plates. The pasteur will pick-up 50 µl or more if
dipped into the conidial suspension and will deliver small inocula when touched
onto an agar surface.
To inoculate liquid medium, use a pasteur pipette and
rubber teat. 1
drop per 50-100 ml is adequate. The whole 1.5 ml suspension is adequate for a
8 l carboy culture.
Note that supplements present in the slope culture will be
leached out into the suspension. This is not normally a serious problem but
for critical work the suspension should be removed from the culture tube as soon
as possible. Even better, pellet the conidia by centrifugation and resuspend
them in a fresh aliquot of sterile water.
Note also that the suspension will contain some mycelial
fragments. If
it is imperative to remove these, filter the suspension through two layers of
sterile muslin held in a filter funnel or in the neck of a flask or over the
mouth of a pasteur pipette as a small sock held in place with autoclave
tape.
III. Large scale conidial suspensions for preparation of
spheroplasts etc.
Inoculate 1 l flasks containing 100 ml of suitably
supplemented Vogel's medium solidified with agar and harvest the conidia by
suspending them in 20 ml sterile water. Filtration through muslin and washing by
centrifugation is usually necessary. Muslin filters are conveniently made by
folding single or double layers into a cone (as for filter paper) and taping
into a funnel. This can be wrapped in foil or paper prior to sterilising.
MYCELIAL FRAGMENTS
Aconidial cultures must be transferred as mycelium. A flamed needle or
spade, cooled in air or in sterile water, is satisfactory for transferring a few
strands of the aerial mycelium to a fresh slope.
Colonies growing on restrictive media in petri dishes are
subcultured by cutting out small pieces of agar
containing some mycelium, using a spade ended needle. A small inoculum is
particularly important when transferring from sorbose agar or the sorbose will
inhibit growth in the new culture! It is convenient to use two spade needles in
rotation, the needle not in use being stood vertically in a holder to cool after
being flamed to red heat.
ISOLATION OF MUTANTS
Mutants resistant to antimetabolites, (e.g. analogous of
metabolites) suppressor mutations, or reverse mutations to prototrophy can be
selected directly by plating conidia treated with a mutagen onto a suitable
selective medium.
Sorbose or cot-1 is used to restrict growth. Auxotrophic
mutations can be selected by filtration enrichment. Mutagen treated
conidia are cultured on liquid Vogel's medium supplemented (if required) to
permit growth of non-mutant conidia. Non-mutant conidia are able to grow and the
resulting colonies can be filtered out of the suspension culture with sterile
muslin or absorbent cotton wool. When all growth has ceased, the remaining
ungerminated conidia are pelleted by centrifugation and plated on medium
supplemented with the specific growth requirement of the mutant sought and grown
under growth restricting conditions using cot-1 or sorbose. Colonies which grow are then tested for the
specific growth requirement. 50% or more of the survivors of the
filtration may be mutants of the desired type, though 5% or less is more
normal.
METHOD
Suspend conidia from a 1.5 ml slope culture in 20 ml
sterile water, filter aseptically through a double layer of muslin, pour into a
petri dish, set shaking, remove lid and irradiate to 95-99% kill with
ultra-violet light.
Since the appropriate dose is likely to vary from strain to strain, an
experiment to determine the necessary doe will be needed first. A convenient way of
shaking the petri dish is to clip it to a vortex mixer and adjust the vibration
speed to produce a standing wave. Pipette the irradiated suspension into 100 ml
of Vogel's minimal (or another appropriate medium) containing 2% sucrose. Incubate with
shaking at 25o, or at 34o if temperature-sensitive mutants are sought. Refilter as often
as necessary until there has been essentially no growth for 24 hours. (It is normally
necessary to filter at 8-12 hour intervals but heavy growth in the period 18-30
hours may require filtrations more frequently to prevent ungerminated conidia
from entangling in and fusing with mycelial growth. When growth has
more or less ceased, refilter and plate the remaining ungerminated conidia onto
supplemented medium.
If cot-1
has been used, this can be done by heating the suspension to 45o adding an equal volume of 45o molten medium containing double strength agar,
sorbose and any supplements. Pour the mixture into plates allow to
solidify, incubate overnight at 25o and then
transfer to 34o for further incubation. If cot-1 has not been
used, the sucrose in the medium must be removed. To do this add a suspension of the conidia
from a 1.5 ml slope, which have been killed by incubating the tube at 60o for several hours, and pellet the conidia by
centrifugation.
Resuspend the conidia in a small volume of sterile water, add to molten
supplemented SGF medium, plate and incubate at 25o. Following a suitable period for growth, pick
colonies of putative mutants into tube of fully supplemented medium prior to
testing for auxotrophy. Alternatively, transfer small inocula to
unsupplemented plates and look for slow growth. Re-pick the slow growing colonies back onto
supplemented medium, grow to conidia and then test fully.
RECOMBINATION ASSAY
When analysing crosses between closely linked genes or
alleles of the same gene, measurement of recombination frequency normally
demands selective plating techniques in which large numbers of ascospores are
plated onto a medium which permits the growth of one
of the two recombinant classes, the prototrophic recombinants. The number of
recombinants may be related either to the total number of ascospores plated or
more conveniently to the total number of viable ascospores. The former can be
estimated by counting the number of ungerminated and germinated spores on an
area of the selective plate and multiplying up to obtain the total count. Viable spores are
estimated by plating out a suitable dilution onto a non-selective medium on
which both the recombinant and parental types can grow and multiplying by the
dilution factor.
Frequently, different numbers of selective and non-selective plates are
used and you will need to take this into account when calculating the prototroph
frequencies. A
convention of this laboratory to express recombination frequency in terms of map
units (% recombination) when considering non-allelic recombination and in terms
of prototrophs per 105 viable ascospores when
considering recombination between alleles of the same gene. The following
method is suitable for estimating prototroph frequencies of between 1 and 1000/105. For each analysis, the following are
required:
.
A mature cross
.
A 28 x 200 mm sterile tube
.
A bottle containing 100 ml sterile water
.
two 100 ml winchesters containing 19 ml minimal layer agar held at 60o
.
one 100 ml
.
5 selective plates
.
3 non-selective plates.
Taking care not to disperse the conidia, add 8 ml of sterile
distilled water to the crossing tube. Vortex mix briefly and pour the spore
suspension through a muslin filter which will retain perithecia, mycelial
fragments and fragments of filter paper. NOTE: over zealous mixing will disrupt the filter
paper. The
fragments may then prove a nuisance in later stages of the analysis. Add a further 8 ml of sterile
water to the crossing tube, re-mix and filter. Repeat if necessary to wash the filter paper
onto the muslin.
Rinse the muslin with the remaining sterile water, collecting all of the
washings into the 28 x 200 mm tube. A sterile spatula is useful for retrieving
the paper and perithecia from the cross tube and for pressing the filter to
dislodge spores. Rinse the spatula in water between crosses then dip in alcohol
and flame sterilise. A water rinse is needed as ascospores remain viable in
ethanol for some time. Ensure the rinsings end up in disinfectant.
Allow the tube to stand for 1-3 hours away from any source
of heat which may cause convection. The spores will settle out to form a black
layer on the bottom of the tube. Decant the supernatant gently into
disinfectant, leaving about 2 ml of ascospore suspension. Swirl the spore
suspension and pour into 19 ml of minimal layer agar held at 60o, this is Bottle A. Incubate for 60 minutes to kill conidia. For the remaining
manipulations, warm the pipettes by flaming prior to use and return the bottles
to 60o as soon as possible. Also, speed is of
the essence since the spore will settle-out rapidly: mixing must immediately
precede each pipetting operation Vortex mix, pipette 1 ml into the
second bottle containing 19 ml of molten minimal layer agar, bottle B 1/20
dilution.
Vortex mix bottle B and pipette 0.5 ml into 19.5 ml of molten minimal
layer agar, bottle C 1/800 dilution. Vortex mix bottle C pipette 3 ml onto each of
3 fully supplemented plates, "C" plates. In cool weather it may be helpful to prewarm
the plates to 60o to aid spreading. Vortex mix bottle A
and pipette 3 ml onto each of 5 selective plates, A plates. For recombination
frequencies in the high range, or if the frequency range is unknown, also plate
from the B bottle onto selective medium.
In most instances, incubate the plates at 25o for 18 hours and transfer to 34o for 24 hours. Count colonies and calculate prototroph
frequencies.
When discarding plates, ensure that they are not left at room temperature
or growth will escape from the plates in a few hours. Keep discard plates
overnight in the 60o incubator or autoclave them
the same day.
When analysing recombination between nit-2 alleles,
incubate A plates for 42 hours at 25o and then
48 hr at 34o.
This method employs 2% sucrose in the layer agar with the
plates containing SGF plates. Excepting in analyses of nit-2 crosses where
SS is used in the A plates. Both parents must
be cot-1. Non cot-1 parents require SGF layer agar and lower
dilutions may have to be used since the colony size will be large. If spore yields are
high or low, it may be desirable to vary the dilutions to give 1/200 to 1/1600
in the C bottle.
When very low recombination frequencies are being measured, the spores
from two or more crossing tube may be combined at the filtration stage. In this case,
reversion rates may become significant and it is desirable to set up control
isoallelic crosses.
CULTURE MEDIA
VEGETATIVE GROWTH
1. Liquid medium
Vogel's 50 X stock
2
ml
Sucrose
2
g
Supplements
as needed
Distilled water
100 ml
(pH should be 5.8)
Autoclave: 10 psi for 10 min. is satisfactory for small volumes (say to
200 ml) but for larger volumes 15 psi for 15 min. may be needed. Give 8 l
carboys at least 20 min.
2. Slopes (make 100 ml per basket of 13 x
100 mm tubes)
Vogel's 50 X stock
2
ml
Sucrose
2
g
Supplements
as needed
Difco Agar
2
g
Note that it is useful to mix the sucrose and agar dry prior to adding
liquids. This helps disperse the agar and reduces problems with lumps and proper
mixing.
Distilled water
100 ml
(pH should be 5.8)
Steam, or autoclave 5 min to dissolve agar. Mix well and
dispense 1.5 ml per tube, taking care not to get agar where it will wet the plug
or the plugs will stick. Plug the tubes with non absorbent cotton. A push plug
formed by inserting a tuft of cotton with forceps is satisfactory. Autoclave at 10 psi
for 10 min (longer if the autoclave load is substantial). Slope the tubes
whilst they cool. The agar should not be more than about half way to the plug or
the mycelium is liable to grow into the plug.
3. Plates (allow 20 ml/plate)
Vogel's 50 X stock
2
ml
Supplements
as needed
Difco agar
2
g
Distilled water
100 ml
(pH should be 5.8)
Autoclave 10 psi 10 min or more as appropriate, then add aseptically:
SGF or SS 20 X stock
5 ml
Mix, cool to less than 50oC and pour into
petri dishes.
Flame surface to break bubbles before agar sets.
4. Layer agar (Make on
day of use)
Vogel's 50 X stock
2
ml
Sucrose
2 g
only for analysis with cot where plates are incubated at 25o then moved to 34o
Difco agar
0.8 g
Supplements
as necessary *
Distilled water
100 ml
Autoclave 10 psi 10 min or 15 psi for larger volumes. Dispense and keep
at 60o in a water bath. * Normally
supplements are not added to layer agar.
CROSSING MEDIUM
1. Tubes
EITHER
Westergaard 4 X stock
25 ml
Sucrose
2 g
Supplements
as needed
Distilled water
75 ml
Adjust pH to 6.5.
OR
SC Medium 2 X stock
50 ml
Sucrose
2 g
Supplements
as needed
Distilled water
50 ml
(pH should be 6.5 without adjustment)
OR
For crosses between nit-2 mutants, use AWM 4 X in place of
Westergaard's.
Dispense 4 ml/tube, containing 6 x 4 cm spill of Whatman # 1 filter paper
folded lengthwise into W. Plug and autoclave 10 psi 10 min or 15 psi 15
min for larger volumes.
2. Plates and tubes for mating type tests
As above but with 2 g Difco agar per 100 ml. Autoclave and
dispense 20 ml per plate or 1.5 ml per tube, plug and slope.
STOCK SOLUTIONS
VOGEL'S MEDIUM N, 50 X STOCK SOLUTION (to make 1 litre)
Dissolve in the order listed. Do not add any
component until the previous one has dissolved completely. Use a magnetic
stirrer.
Gentle heat may be used. BDH "analar" chemicals are recommended.
Distilled water
700 ml
Na3 citrate. 5½ H20
150 g (.2H20, 130 g)
KH2PO4
anhydrous
250 g
NH4NO3
anhydrous
100 g
MgSO4 . 7H20
10 g
CaCl2 . 2H20
5
g
dissolved in 50 ml distilled water
Trace element solution
5
ml
Biotin
25mg
Chloroform
2 ml
(as a preservative)
(this will be pH 5.8 when diluted. Do not adjust pH of stock)
Store at room temperature in a dark bottle. Check for presence
of preservative from time to time.
TRACE ELEMENT SOLUTION
Dissolve in the order listed. Use BDH "analar"
chemicals where possible.
Distilled water
95 ml
Citric acid 1H20
5 g
ZnSO4.7H20
5 g
Fe(NH4)2(SO4)2.6H20
1 g
CuSO4.5H20
0.25 g
MnSO4.1H20
0.05 g
H3BO3
anhyd
0.05 g
Na2MoO4 .
2H20
0.05 g
Chloroform
1 ml (as a preservative)
Store refrigerated in a dark bottle.
SS 20 X STOCK SOLUTION
Sorbose
20 g
Sucrose
1
g
Distilled water
100 ml
Autoclave 10 psi 10 min. Store refrigerated.
SS (nit-2) 20 X STOCK SOLUTION
for use when plating cross homozygous nit-2 and am-1.
Sorbose
10 g
Sucrose
2
g
Distilled water
100 ml
Autoclave 10 psi, 10 min. Store refrigerated.
SGF 20 X STOCK SOLUTION
Sorbose
20 g
Glucose
0.5
g
Fructose
0.5
g
Distilled water
100 ml
Autoclave 10 psi, 10 min. Store refrigerated.
WESTERGAARD MEDIUM, 4 X STOCK SOLUTION
KNO3
4 g
KH2PO4
4 g
MgSO4 . 7H20
4.1 g
CaCl2 . 6H20
0.8 g (.2H20, 0.54 g)
NaCl
0.4 g
Trace element solution
0.4 ml
Biotin
10
mg
Distilled water
1 litre
Chloroform
2 ml (as a preservative)
Store at room temperature in a dark bottle. Check for presence
of preservative from time to time.
SC MEDIUM 2 X STOCK
KNO3
2.0 g
K2HPO4
1.4 g
KH2PO4
1.0 g
MgSO4 . 7H20
2.0 g
NaCl
0.2 g
CaCl2
0.2 g
Biotin
10 mg
Trace element solution
0.2 ml
Distilled water
1 l
Chloroform
2 ml
Store refrigerated in a dark bottle.
AWM 4 X STOCK SOLUTION for use in crosses homozygous nit-2.
NH4NO3
2 g
KH2 PO4
4 g
MgSO4 . 7H20
4.1 g
CaCl2 . 6H20
0.8 g (.2H20, 0.54 g)
NaCl
0.4 g
Trace element solution
0.4 g
Biotin
10mg
Distilled water
1 litre
Chloroform
2 ml (as a preservative)
Store in a dark bottle at room temperature. Shelf life beyond 1
month not proven.
SUPPLEMENTS
stock solutions*
mg/100 ml medium mg/100 ml drops/1.5 ml slope
adenine
20
adenine sulphate
30
adenosine**
40
500
1
L-alanine
50
400
5
p-aminobenzoic acid
0.2
3
2
anthranilic acid
13
arginine
50
asparagine
30
400
1
(in presence of alanine)
20
choline chloride
38
L-cysteine HCl
20
glycine
150
L-histidine
20
800
1
L-homoserine
20
indole
2
inositol
5
100
1
L-isoleucine
23
L-leucine
20
L-lysine
40
600
2
L-methionine
50
monosodium glutamate
40
nicotinamide
1
D-pantothenate
1
L-phenylalanine
20
L-proline
50
pyridoxin HCl
1
L-serine
20
300
2
sulphanilamide
3.5
thiamine (aneurin)
1
L-threonine
10
L-tryptophan
40
L-tyrosine
20
uracil
10
uridine
10
150
2
L-valine
20
![]()
*
autoclave and refrigerate
** best for
vegetative growth; adenine is better for crosses.