Introduction
to Neurospora biology and genetics,
a cytological
perspective
Namboori B.
Raju
June 2003
Shear
and Dodge (1927 J. Agr. Res., 34:1019-1042) discovered mating types (mat A and mat a) in Neurospora, and
described the life histories of two eight-spored heterothallic species (N.crassa and N. sitophila) and one four-spored homothallic species (N. tetrasperma, later called
pseudohomothallic). They named the genus Neurospora
because of the nerve-like ornamentation (striations) on developing ascospore
walls (Fig. 1).
.
Fig. 1.
Ascospore ornamentation in N. crassa, hence the genus name Neurospora.
Dodge (1927 J. Agr. Res., 35:289-305)
described the nuclear basis for heterothallism in the eight-spored species and
for pseudohomothallism in the four-spored species. In N. tetrasperma, ascus development is programmed so that each of the
four ascospores encloses two nuclei of opposite mating type; single-ascospore
cultures are thus self-fertile (see also Raju 1992a Mycol. Res. 96:103-116;
Raju and Perkins 1994 Dev. Genet. 15: 104-118).
In the eight-spored N. crassa,
the linearly ordered ascospore pairs reflect the underlying genetic events
during meiosis, and provide the clearest visual demonstration that crossing
over occurs at the four-strand stage during meiotic prophase. When a gene
marker is close to the centromere (no crossing over between the gene and
centromere), the two alleles are likely to segregate from one another at the
first division of meiosis and result in a 4:4 ascospore pattern for the
segregating alleles. In contrast, when a gene marker is far from the centromere
(cross over likely) the two alleles often segregate at the second division of
meiosis, and such asci show a 2:2:2:2 or 2:4:2 ascospore pattern for the
segregating alleles (Fig. 2).

Fig.2. A rosette of maturing asci from a cross heterozygous for cys-3 having pleiotropic effects on ascospore maturation and on cysteine biosynthesis. Mature asci show 4 black and 4 white spores. The white spores received the cys-3 mutant allele. Asci with all eight spores unpigmented are immature. One ascus at top center and two asci at upper left show first-division segregation. The remaining mature asci show second-division segregation patterns resulting from crossing over between cys-3 and centromere.
B.O.
Dodge also publicized the virtues of N.
crassa for genetic studies, and it was Dodge (then at New York Botanical
Garden) who provided the Neurospora
strains to Stanford’s George Beadle and Edward Tatum for their 1941 landmark
studies, linking gene mutations with nutritional requirements -- later came to
be known as one-gene one-enzyme hypothesis for which they were awarded Nobel
Prize in 1958. With these humble beginnings, Neurospora became the model filamentous fungus for numerous subsequent
discoveries on vegetative incompatibility, recombination, gene conversion,
intragenic complementation, biological clocks, repeat-induced point mutations,
signal transduction, chromosome rearrangements, meiotic drive, population
biology, and post-transcriptional gene silencing (see Perkins 1992, Genetics
130: 687-701; Davis and Perkins 2002, Nature Reviews Genetics 3:397-403). For
general information on Neurospora
genetics and genes, see
Dodge (1927) initiated the early
studies on ascus development, and Barbara McClintock (1945, Amer. J. Bot. 32:
671-678) showed that meiosis and chromosome behavior in Neurospora are very similar to that of higher plants and animals
(see Singleton 1953 Amer. J. Bot 40: 124-144). McClintock, Singleton, and E.G.
Barry (at Stanford) have skillfully used an aceto-orcein staining procedure for
studying meiotic chromosome behavior. I use an iron-hematoxylin staining
procedure (Raju and Newmeyer 1977 Exp. Mycol. 1: 152-165), which stains
chromosomes, nucleolus, spindles, spindle pole bodies, and ascus apical pore
very well. A ten-fold dilution of ferric acetate and hematoxylin solutions is
also good for preparing unfixed rosettes of maturing asci for photography
(Figs. 2-5). The DNA-specific acriflavin “staining” and fluorescence microscopy
have also been used for detailed meiotic chromosome analysis (Raju 1986
Mycologia 78: 901-906).

Fig. 3. Wild type x Wild type. Normally maturing asci in a cross between two unrelated wild type strains. Inbred parents would produce many aborted asci.
We routinely grow the protoperithecial
parent (female) on Petri plates containing synthetic crossing medium for 5 days
(at 25 C) and fertilize with conidia (or mycelial fragments) from a strain of
opposite mating type. Developing perithecia contain
ascogenous hyphae
and young asci at 3 days, various meiotic division stages at 4-5 days, spore
delimitation and maturation from 5-8 days. Mature ascospores are ejected
forcefully shortly there after. A perithecium of N. crassa produces well over
200
asynchronously
developing asci. Karyogamy in the young ascus results in a diploid zygote
nucleus, which immediately undergoes meiosis (two divisions) and a post meiotic
mitosis. The spindles at the first and the second divisions are aligned
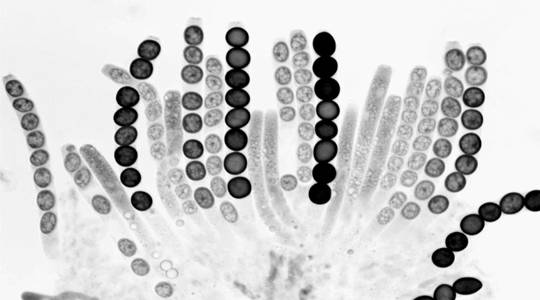
Fig.
4. Wild type x Round spore. A rosette of maturing asci. All eight
ascospores (R as well as R+) are round, because the Round-spore
mutation is ascus dominant.
longitudinally and they are also well
spaced at the second division. The third-division spindles are aligned across
the ascus, but all eight nuclei line up in single file in the elongated narrow
ascus prior to ascospore delimitation. Another mitosis occurs in the young
ascospores and four or five additional mitoses occur in the mature black
ascospores (Fig. 6). An illustrated account of meiosis and ascospore genesis is
given in Raju (1980, Eur. J. Cell Biol. 23: 208-223). Many gene mutations
result in defective
meiosis, abnormal ascus or ascospore
morphology and size (reviewed in Raju 1992b Mycol. Res. 96: 241-262).
Turner and Perkins (1979, Genetics 93:
587-606) discovered Spore killers in Neurospora
that behave like meiotic drive elements in Drosophila.
When heterozygous (Killer x Sensitive), meiosis and ascospore delimitation are
normal, but every ascus contains four large, black, viable ascospores and four
small, white, dead ascospores; all viable ascospores carry the killer allele
(Fig. 5). Thus spore killers distort genetic ratios of Sk-linked genes. Sensitive nuclei are rescued when they are
enclosed together with one or more killer nuclei in the same giant ascospore
(Raju 1979 Genetics 93: 607-623; 1994 Mycologia 86: 461-473; Raju and Perkins
1991 Genetics 129: 25-37). David Perkins has single handedly analyzed more than
350 chromosome rearrangements in N. crassa, and showed their usefulness
for gene mapping and for studies on heterokaryon incompatibility.
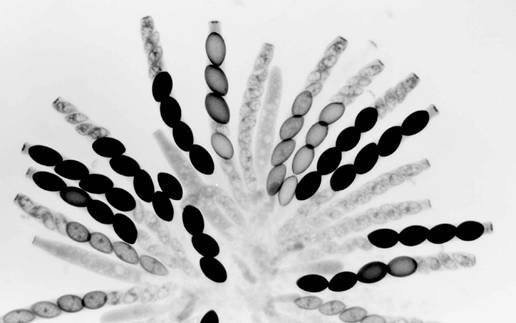
Fig.
5. Sk-2K x Sk-2S. A rosette of maturing asci from a cross
heterozygous for Spore killer-2. Most asci show four normal (larger) spores and
four small aborted spores. The few asci not showing the 4:4 pattern are still
immature. All the maturing ascospores are Sk-2K. The first-division segregation (4K:4S) in all
asci indicates that there is no crossing over between Spore killer-2 and the
centromere.
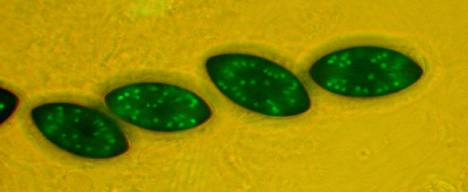
Fig. 6. Mature black
ascospores contain up to 64 nuclei resulting from 4-5 mitoses.
Figs. 6-9. The nuclei are tagged by fusing the chromosomal protein
gene histone H1 with the green fluorescent protein (GFP) gene from a jellyfish.
In the last three years, I have
provided cytological support for novel (and more trendy) studies on meiotic
gene silencing, and expression of GFP-tagged genes during ascus development.
Shiu et al. (2001, Cell 107: 905-915) have shown that an unpaired
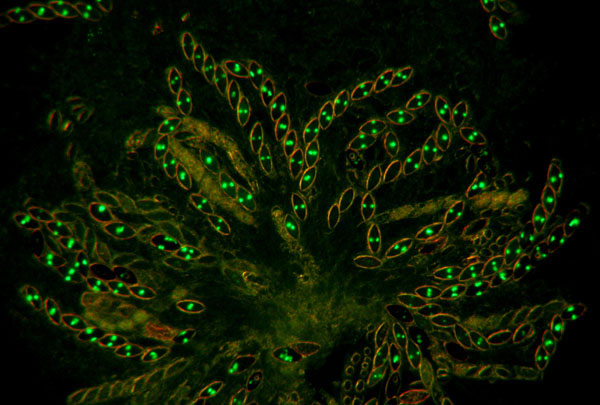
Fig.
7. hH1-GFP x hH1-GFP. A rosette of
maturing asci at eight days after fertilization. All eight ascospores show glowing nuclei; the
ascospores are binucleate at this stage. When homozygous, histone H1 is
expressed throughout meiosis and ascospore maturation.
ectopically inserted gene, whose
function is essential for meiosis, triggers post- transcriptional gene
silencing when made heterozygous in a cross. The silencing occurs not only of
the duplicated ectopic gene but also of any other paired or unpaired gene sequence(s)
elsewhere that are homologous to that of the ectopic gene (see Kasbekar
2002 J. Biosciences, for a
commentary). Ascus development in such
heterozygous crosses is arrested at a characteristic stage reflecting the
function of the inserted gene. Homozygous crosses carrying the same ectopic
gene insert do not show silencing and develop normally. We have demonstrated
this novel phenomenon using several cloned genes whose functions are known to
be required for normal meiosis or ascus development. With the recent use of
GFP-tagged histone H1 and b-tubulin genes, we are able to visualize the
expression or silencing of these genes during ascus development in homozygous
(Figs. 7) and heterozygous crosses (Figs. 8, 9), respectively. The meiotic
silencing by unpaired DNA (MSUD) in heterozygous asci does not extend into
autonomously developing ascospores. Thus, in a heterozygous cross of hH1-GFP x wild type, four of the eight
ascospores in each ascus begin to show hH1-GFP
expression (glowing nuclei) approximately12-24 hours after spore delimitation
(Fig. 9). Apparently, the MSUD in heterozygous asci is a defense mechanism
against invading foreign DNA.
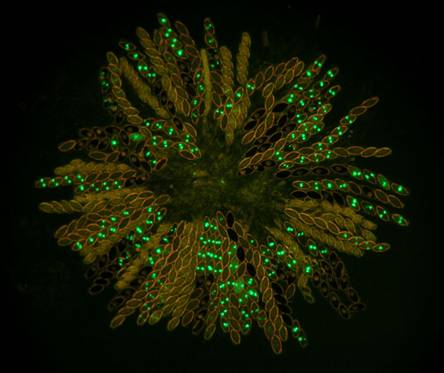
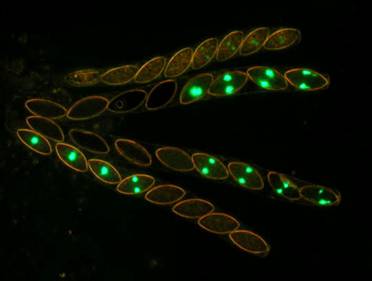
Figs.
8. Wild type x hH1-GFP. Histone H1-GFP is completely silenced in the developing
heterozygous asci until after ascospores are cut out. However, the silencing
does not extend into autonomously developing ascospores. Four of the eight
ascospores in each ascus show the glowing nuclei.
Fig. 9. Wild type x hH1-GFP. Four asci at a higher magnification. The top ascus is just beginning to show the reactivation of the silenced hH1-GFP gene.