We report the secretion of amylase by A. nidulans R153 and repression of its expression by various carbon sources. Although amylases from several species of Aspergillus have been characterized and two alpha-amylase genes from A. niger var. awamori (Korman et al. 1990. Curr. Genet. 17:203-212.), as well as three alpha-amylase genes from A. oryzae (Wirsel et al. 1989. Mol. Microbiol. 3:3-14) have been cloned and sequenced, an extensive search of the literature uncovered reference only to beta-amylase in a wild strain of A. nidulans (Chatterjee and Das 1988. Biotech. Lett. 10:143-147). We examined A. nidulans R153 for amylase secretion using a convenient starch agar plate assay. We found evidence not only for amylase secretion but also for regulation of enzyme synthesis and/or secretion through catabolite repression.
A. nidulans R153 (wA3; pyrA4) conidia were streaked on 1.5% agar plates containing 3% soluble potato starch, prepared by the Lintner method (Lintner, C.J. 1888 J. prakt. Chem. 36:481; Sigma, St. Louis, MO, catalog no. S2630), 11 mM KH2PO4, 10 mM (NH4)2HPO4, 7 mM KCl, 2 mM MgSO4, trace elements (Cove, D.J. 1966 Biochim. Biophys. Acta 113:51-56), 0.0001% pyridoxine, and 0.005% ampicillin. When media were supplemented with additional carbon sources, the following were added: 2% dextrose, 1.3% ethanol, 0.04% fructose, 50 mM glycerol, or 50 mM threonine. The plates were incubated at 37°C for 48 hrs. and then stored at 4°C for 48 hrs. to precipitate undigested starch. A clear zone surrounding the colonies was interpreted as evidence of amylase secretion.
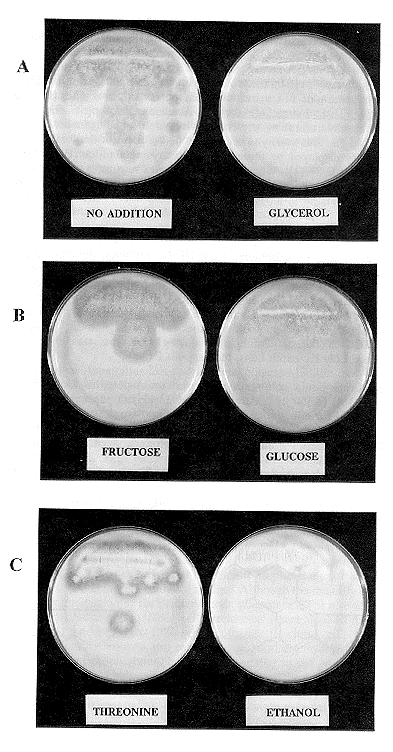
A. nidulans R153 conidia germinated, developed mycelia and hyphae and formed conidia on all media tested, including medium containing starch as the sole source of carbon. As shown in Figure 1, amylase secretion was detected on plates containing starch as the sole carbon source (1A, left), but not in starch media supplemented with glycerol (1A, right). No amylase secretion was detected when the medium was supplemented with glucose (1B), or ethanol (1C) indicating that the presence of these carbon sources inhibited the production and/or secretion of amylase. Neither fructose (1B) nor threonine (1C) inhibited amylase expression. The lack of amylase secretion in starch media containing glucose, ethanol, or glycerol is consistent with the regulation of amylase expression by carbon catabolite repression rather than induction by starch. Repression in the presence of these carbon sources may be due to the expression of the creA gene product, a putative DNA binding protein which acts as a global regulator of carbon catabolite repression (Dowzer and Kelly 1991. Molec. Cell Biol. 11:5701-5709).
Figure 1. Effect of carbon sources on amylase expression by A. nidulans. A suspension of R153 conidia was streaked on plates containing 3% soluble starch with the following additions: (A) none (left) and glycerol (right); (B) fructose (left) and glucose (right); (C) threonine (left) and ethanol (right). The plates were refrigerated to allow starch to form a white precipitate. The presence of clear zones surrounding the colonies on the plates on the left in A,B, and C is interpreted as evidence of amylase secretion. No clear zones are seen in plates on the right .
