Detection of pterins and enzymatic activities of GTP-cyclohydrolase I and sepiapterin
reductase in Neurospora crassa and Phycomyces blakesleeanus
J. Maier and H. Ninnemann - Institut für Chemische Pflanzenphysiologie, Univ. Tübingen,
Corrensstr. 41, 7400 Tübingen, Germany
The best known pterin in Neurospora crassa is molybdopterin, which is a constituent
of the flavohemoenzyme nitrate reductase and participates in blue-light promoted
conidiation (Klemm and Ninnemann 1979 Photochem. Photobiol. 29:629-632, Ninnemann
1991 J. Photochem. Photobiol. 9B:189-199). There is evidence that additional pterin
derivative(s) occur in N. crassa mycelium (Siefermann-Harms, Fritz and Ninnemann 1985
Photochem. Photobiol. 42:771-778) as well as in crude extracts of the zygomycete
Phycomyces blakesleeanus (Kiewisch and Fukshansky 1991 Photochem. Photobiol. 53:407-
409). For this reason, we have purified and characterized unconjugated pterins from
mycelium of N. crassa and from mycelium and sporangiophores of P. blakesleeanus.
In addition we have determined the activities of two related enzymes: GTP-
cyclohydrolase I and sepiapterin reductase. GTP-cyclohydrolase I converts GTP to
dihydroneopterin triphosphate, which is the precursor for unconjugated pterins and folates,
but not for molybdopterin. Sepiapterin reductase catalyzes the last step in biosynthesis of
biopterin from GTP and has previously been reported only in vertebrates and arthropods
(for review see Brown 1985 In: Folates and Pterins. Vol. 2. Blakely and Benkovic eds., John
Wiley and Sons, New York, pp. 115-154; Nichol, Smith and Duch 1985 Ann. Rev. Biochem.
54:729-764; Duch and Smith 1991 J. Nutr. Biochem. 2:411-423).
N. crassa wild type (strain 987 from the Fungal Genetics Stock Center, University of
Kansas) was grown on nitrate reductase-inductive Vogel's medium (with 10g/l glucose)
modified so that 50 mM NaNO3 was the only nitrogen source. P. blakesleeanus (strain F813,
wild type, +-strain, a generous gift from Prof. Dr. Oberwinkler, Univ. Tübingen, Germany)
was grown on malt medium (2% malt extract) in 300 ml flasks for the production of
mycelium or with additional 1.2% agar in Petri dishes for the production of sporangiophores
(state V).
The mycelia were harvested by filtration through nylon meshes. About 0.5 g of
frozen sample was triturated in the dark under N2 to a fine powder and solubilized with 1
ml of 0.1 N HCl. Pterins were analyzed by cation-exchange chromatography and HPLC
determination as described initially by Fukushima and Nixon 1980 Anal. Biochem. 102:176-
188 and as modified by Ziegler 1985 J. Cell. Biochem. 28:197-206. The following
modifications were used: monapterin was added as an internal standard prior to the
oxidation step and pterins were eluted from the cation-exchange resin with 1 M NH4-acetate
dissolved in a 1:4 mixture (v/v) of methanol and water. The following instrumentation
assembly was used for the HPLC analysis: Pharmacia pump 2248, Pheodyne loop injector
(20 ul); Hypersil ODS C-18 column (5 um particle size, 4.6 x 250 mm) and pre-column (4.6
x 20 mm), Shimadzu fluorescence monitor type RF-551 and Spectraphysics integrator
ChromJet. Identification of pterins was done by co-chromatography with the respective
standard pterins (Schircks Laboratories, Jona, Switzerland) in different solvent systems as
described by Ziegler 1985 J. Cell. Biochem. 28:197-206. Pterins were quantified by
fluorescence yield comparison with the corresponding standards in concentrations within the
linear response range of the fluorescence monitor.
Protein extracts for enzyme tests were made by sonification of the harvested mycelia
or sporangiophores in 2.5 mM EDTA, 50 mM Tris-HCl (pH 8.0). After centrifugation at
12,000 g for 5 min the supernatant was desalted on Sephadex G-25 columns (NAP-5,
Pharmacia, Freiburg, Germany) equilibrated with the sonification buffer. Protein
concentrations were estimated by the Coomassie blue dye assay (Bio-Rad, München,
Germany), with bovine serum albumin as standard. Enzyme tests for GTP-cyclohydrolase
I and sepiapterin reductase were performed as described by Kerler et al. 1990 J. Cell.
Physiol. 142:268-271.
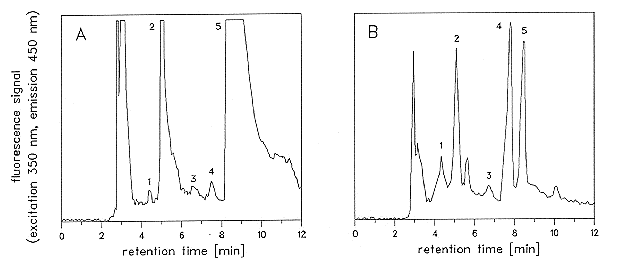
Figure 1. Elution profile for pterins isolated from Neurospora crassa (A) and Phycomyces blakesleeanus (B).
1) neopterin; 2) monapterin (added as internal standard); 3) xanthopterin; 4) biopterin; 5) 6-
hydroxymethylpterin. For peak quantification we diluted the sample until the peak area fitted in the linear range of a standard curve.
Figure 1 shows elution profiles of pterins from N. crassa and P. blakesleeanus. The
dominant unconjugated pterin in N. crassa was found to be 6-hydroxymethylpterin,
accompanied by minor amounts of neopterin and biopterin (Figure 1A and Table 1). In P.
blakesleeanus, the main pterin was biopterin. It was present in the same concentration in
mycelium and in sporangiophores; in addition neopterin, xanthopterin and 6-
hydroxymethylpterin were present (Figure 1B and Table 1). GTP-cyclohydrolase I activities
were 7.6 times higher in mycelia of N. crassa than in mycelia of P. blakesleeanus and was not
found in sporangiophores of P. blakesleeanus (Table 1). Sepiapterin reductase showed a
different activity pattern: it was high in P. blakesleeanus and low in N. crassa (Table 1).
GTP-cyclohydrolase I produces dihydroneopterin triphosphate from GTP, the
precursor for the biosynthesis of pterins. The product is dephosphorylated to
dihydroneopterin and yields, after removal of a C2-residue from the C3-side chain 6-
hydroxymethyldihydropterin, which is the precursor for folate biosynthesis. The reduced
pterins were detected after oxidation to fluorescent compounds. Analysis of N. crassa
revealed large amounts of 6-hydroxymethylpterin, accompanied by a high GTP-
cyclohydrolase I activity. In P. blakesleeanus the total amounts of pterins were decreased
and the level of GTP-cyclohydrolase I was also relative to those in N. crassa. In
sporangiophores of P. blakesleeanus no GTP-cyclohydrolase I was detectable; the amounts
of neopterin and 6-hydroxymethylpterin were decreased compared with mycelia of P.
blakesleeanus.
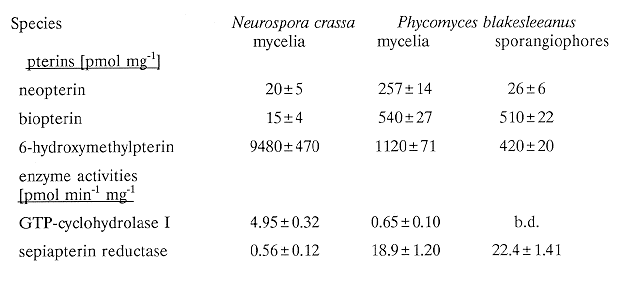
Table 1. Estimation of pterin concentrations (per mg wet weight of mycelia or
sporangiophores) and activities of GTP-cyclohydrolase I and sepiapterin reductase (per mg
of soluble protein in crude extracts after desalting with Sephadex G-25). b.d.= below
detection limit. Values are means of three experiments.
In animals dihydroneopterin triphosphate is converted to 6-pyruvoyl tetrahydropterin,
which is sequentially reduced by the action of sepiapterin reductase to yield
tetrahydrobiopterin. Interestingly, in fungi, sepiapterin reductase was detectable and its
activity seems to correlate with the amount of biopterin (Table 1): mycelia of N. crassa
showed low concentrations of biopterin and low activities of sepiapterin reductase, for
mycelia and sporangiophores of P. blakesleeanus higher activities of sepiapterin reductase
corresponded with higher amounts of biopterin.
The financial support of the Deutsche Forschungsgemeinschaft is acknowledged.

