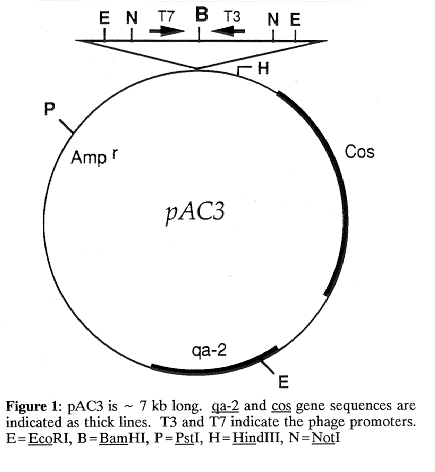
Gene cloning in Neurospora crassa is often achieved by mutant complementation. However, the cloning strategy sometimes requires the isolation of a specific genomic region (by chromosome walking) before transformation of N. crassa . This is the case, for example, if the gene to be isolated has a non-selectable phenotype. Here we specifically describe the construction of the cosmid vector, pAC3, which is designed for direct transformation of N. crassa, its utilization for the construction of a genomic library, and chromosome walking in the region of un-10 on linkage group VII.

We used the pAC3 vector to construct the CBM1 N. crassa genomic library. We isolated partially digested chromosomal DNA directly from 74A N. crassa agarose plugs prepared as for pulse field electrophoresis (Ballario et al. 1989. Fungal Genetics Newsletter 36:38). The chromosomal DNA was dephosphorylated and ligated at a pAC3 arms-insert ratio of 3:1 (see below for the definition of arms). To avoid the loss of methylated sequences (Grant et al. 1990. Proc. Natl. Acad. Sci. 87:4645) and the recombination events between repeated sequences, a Gigapack Gold packaging extract (Catalogue no. 200214 from Stratagene) was used for packaging and E. coli PLK-F' cells (recA lac mcrA mcrB hsdR gal supE [F' proAB lacqZ M15 Tn10 (tetr)]) were infected. An efficiency of about 1.2 x 10(5) clones per ug of N. crassa chromosomal DNA was obtained.
2) The origin of replication of pJB8, present in pAC3, maintains the cosmid at a low copy number and may permit survival of clones containing sequences toxic for E. coli.
3) The presence of T3 and T7 phage promoters flanking the cloning site allows both the preparation of RNA probes representing the ends of the cosmids and restriction mapping using T3 and T7 DNA sequencing primers (Evans et al. 1989. Gene 79:9).
4) The sites for a rare cutter restriction enzyme (NotI) flanking the cloning site make it possible to remove the inserts and facilitate physical restriction mapping.
5) A selectable marker for direct selection of N. crassa transformants has been included.
We have used the CBM1 library for a chromosome walk from un-10 toward white collar 1 (wc-1) on the right arm of linkage group VII. As a starting point for the walk, the cosmid clone 10:E12 from the Vollmer-Yanofsky (V.Y.) library complementing the un-10 mutation was used. In the absence of indications about the genomic orientation of cosmid 10:E12, we were forced to walk in both directions. Fragments A and B (in Fig. 2) of cosmid 10:E12 were identified by conventional restriction mapping and Southern hybridization and used as starting points in the walk.
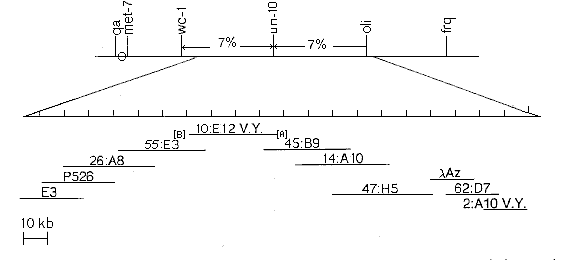
Fig. 2. Chromosome walking from un-10 toward wc-1 and oli. Part of the linkage groups VII map is shown on the top, the genetic distances in the region between wc-1 and oli are indicated. The inserts of the walking cosmids and lambda clone are shown as horizontal lines below the chromosome. The cosmids indicated with the symbol V.Y. are from the Vollmer-Yanofsky library, the others are from CBM1. Probes A and B, used to start the walks, are indicated by boxes. Cosmid 10:E12 was kindly provided by T. Schmidhauser. The clone Az was isolated from the lambda J1 library (M. Orbach) available from FGSC.
Starting from probe A (Fig. 2) we made four steps of walking, covering about 120 kb, and finally found the cosmid 62:D7 from CBM1 overlapping the 2:A10 cosmid from the V.Y. library. This clone was previously mapped at the extreme left of oli by McClung et al. (1989. Nature 339:558) and revealed the walking orientation toward frq. The isolation of the last cosmid (62:D7) was preceded by a walking step in a Neurospora lambda library to isolate the clone Az. This step was necessary to cover about 4 kb apparently absent in both the V.Y. library and ours.
Starting from the probe B we have made four steps so far, representing about 80 kb of genomic sequences in the direction of wc-1. All the walking steps have been checked with the RFLP mapping technique (done by A. Folgori) always confirming the assignment of the selected cosmids to the correct linkage group VII region.
This chromosome walking in the CBM1 library, together with that done by McClung et al., results in the cloning of about 390 kb from the right arm of linkage group VII represented by a series of contiguous cosmids. Having this large region already cloned could allow the molecular investigation of the several known genetic markers mapped in the region.
The authors wish to thank Dr. G. Morelli for scientific discussion; Robert Geever who generously provided us with the MSK338 plasmid; T. Aversa and M. Helmer Citterich for skillful technical assistance. This work was partially supported by the Fondazione Pasteur-Cenci Bolognetti and by Piano Nazionale Tecnologie Avanzate Applicate alle Piante, Ministero Agricoltura e Foreste.