Ascospore segregants ("slime"-like) of the triple mutant fz(fuzzy);sg(spontaneous germination) os-1(osmotic) ("slime"; Emerson 1963. Genetica 34:162-182) of Neurospora crassa germinate as a plasmodium which, after some time, results in a morphologically abnormal mycelium. If the mycelium of a "slime"-like isolate is cultured under high osmotic pressure (Nelson et al. 1975. Neurospora Newsl. 22:15-16), it releases cells lacking walls which proliferate as spheroplasts. Comparative studies on the production of carbon-catabolic exoenzymes in mycelial and plasmodioid phenotypes of "slime" suggest that the cell wall may have a role in the transduction of environmental signals which affect the synthesis and secretion of exoenzymes (for a review see Terenzi et al. 1990. Fungal Genetics Newsl. 37:47-49).
It is also known that cell wall synthesis inhibitors such as sorbose or polyoxin B (Selitrennikoff and Zucker 1982. Exper. Mycol. 6:65-70) inhibit the growth of wild type mycelium of N. crassa, but not that of "slime" spheroplasts. These results suggest that cell wall assembly may be a growth- limiting process in mycelial strains but not in wall-less ones. Along this line, we now show evidence suggesting that cell wall assembly may be involved in the phenotypic effect of mutations which cause growth inhibition either under high osmotic pressure (os-1) or high temperature (cot-1). The tester strain RCP-34 (A;fz;sg;os-1 al-1;cot-1;nic-3) was obtained from a cross of a "slime"-containing heterokaryon, FGSC 2713 (A;fz;sg;os-1 arg-1 al-1 cr-1) + (a;tol-1 pan-1) and BAT 9-5 (a;cot-1; nic-3). The mycelial phenotype (mycelial intermediate) and the plasmodioid phenotype (stable "slime") of segregant RCP-34 were obtained as described by Pietro et al. (1990. J. Gen. Microbiol. 136:121- 129). These strains were cultured in liquid Vogel's complete medium, with shaking, at different concentrations of NaCl or sorbitol to test the expression of the os-1 phenotype, or either at 25 C or 35 C to test the expression of the cot-1 phenotype. Strains FGSC 424 (wild type), FGSC 810 (os-1) and BAT 9-5 (nic-3;cot-1) served as controls.
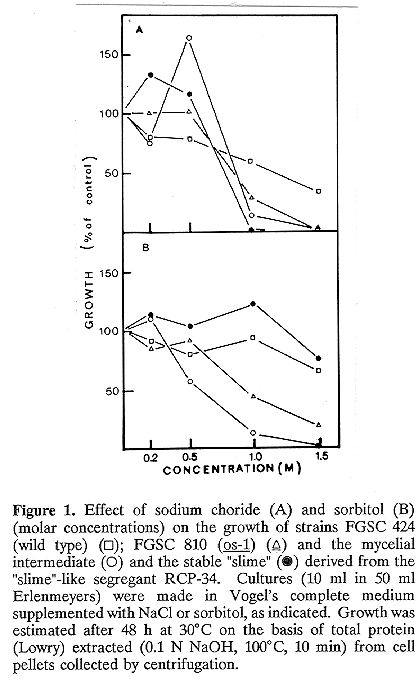
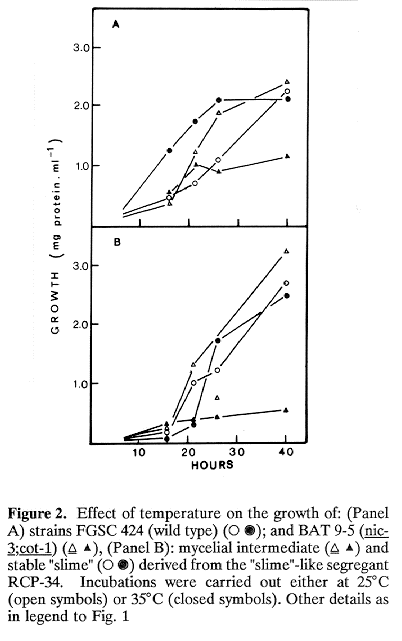
Figure 1A shows that increasing concentrations of NaCl inhibited the growth of the two phenotypes of RCP-34 and the os-1 control as well. On the other hand, increasing sorbitol concentrations (Figure 1B) inhibited the mycelial intermediate of RCP-34 and the os-1 control, but not the stable "slime" derived from RCP-34 mycelium. This result confirmed previous observations (Pietro et al. 1990). A possible explanation for the different effects of NaCl and sorbitol on the stable "slime" RCP-34 is that NaCl, but not sorbitol, besides producing an osmotic stress, produces also an ionic stress to which "slime" spheroplasts may be sensitive. The effect of temperature on the growth of a cot-1 strain is shown in Figure 2A. After an initial lag period of approximately 16 hours, the growth rate of the mutant at 35 C diminished sharply. The same was true for the mycelial intermediate of strain RCP-34 (Figure 2B) and, in this case, the inhibitory effect temperature was rather striking, as it also was for colonies formed at 35 C on solid medium, which were much smaller than those of the cot-1 control (not shown). In contrast, the phenotypic character of the cot-1 mutation (restricted growth at 35 C) was not observed for the stable "slime" spheroplasts derived from isolate RCP-34, which proliferated actively at this temperature.
The primary biochemical defect of either os-1 or cot-1 has not yet been characterized. It has been suggested that the os-1+ gene may play a role in cell wall assembly (Selitrennikoff et al. 1981. Exper. Mycol. 5:155-160) and this suggestion may apply for the cot-1+ gene as well. A possible explanation for the inhibitory effects of osmolarity or temperature, respectively, on the growth of os-1 and cot-1 mutants is that, under restrictive conditions, a defective rate of cell wall formation becomes limiting for the rate of growth. The absence of effects of os-1 and cot-1 mutations in stable "slimes" reinforces this idea, and support a suggestion made by several authors (e.g. Kang and Cabib 1986. Proc. Natl. Acad. Sci. USA 83:5808-5812) that in fungal cells, cell wall assembly and cell growth are functionally coupled. Supported by FAPESP, FINEP, CNPq and CAPES.

