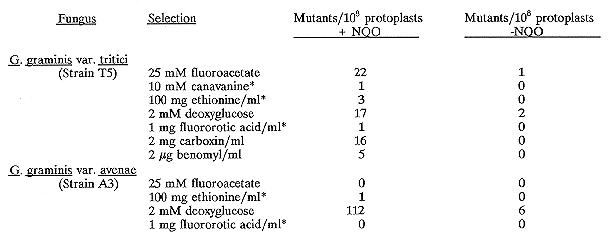
* The figures here represent the mean from two or more experiments
The ascomycete fungus Gaeumannomyces graminis is the causative agent of take-all disease of cereals. Much information about the physiology and pathology of this organism has been generated (Asher and Shipton (Eds.) "Biology and Control of Take-All", Academic Press, 1981), but genetic studies such as the production of mutants have been hindered by problems in obtaining viable propagules suitable for mutagenesis (Blanch et al. 1981. Trans. Brit. Mycol. Soc. 77:391-399). The fungus is homothallic but many strains cannot be induced to form perithecia in culture and even the fertile strains produce insufficient numbers of ascospores for use in mutagenesis. It is, however, possible to produce and regenerate large numbers of protoplasts and Rochefrette et al. (1979. Ann. Phytopath. 11:43-51) reported the mutagenesis of G. graminis protoplasts using nitrosoguanidine (NTG). Here we report the mutagenesis of protoplasts by 4-nitroquinolene oxide (NQO), reportedly a safer and more disposable mutagen than NTG (Bal et al. 1977. Mutat. Res. 56:153-156).
Protoplasts were prepared by standard methods (e.g. Ballance and Turner 1985. Gene 36:321-331) with the following modifications. Protoplasts were produced from washed 2-day old liquid cultures (potato dextrose broth) by shaking (100 rpm) in 0.6 M KCl, 5 mg Novozyme 234/ml for 4 h at 25 C. Mycelial debris was filtered out with four layers of Miracloth (Calbiochem Inc, USA) and the resulting suspension pelleted by centrifugation at 3000 x g. The supernatant was removed and the protoplasts were washed by resuspending them in 0.6 M KCl and repelleting them three times.
Approximately 108 protoplasts were incubated for 1 h at room temperatures in 5 ml 0.6 M KCl containing NQO (1 ug/ml Sigma) from a 10 mg/ml stock in acetone (this treatment was predicted to kill approximately 99% of protoplasts; see Figure 1). After treatment, the NQO was neutralized by the addition of an equal volume of 5% sodium thiosulphate in 0.6 M KCl, protoplasts were washed three times, as previously described, in protoplast regeneration medium (per liter: NaNO3, 4 g; KCl, 44g; KH2PO4, 1.52 g; MgSO4.7H2O, 1 g; glucose, 10 g; yeast extract, 0.5 g; casamino acids, 0.2 g; FeSO4.7H2O, 1 mg; ZnSO4.7H2O, 10 mg; CuSO4.5H2O, 1 mg; Na2B4O7.10H2O, 1 mg; and (NH4)6Mo7O24.4H2O, 50 mg; adjusted to pH 6.5) and added to 5 ml molten regeneration medium containing 0.5% agar and the appropriate inhibitor, which had been cooled to 48 C. The protoplast/agar mix was then overlayed onto protoplast regeneration agar plates.
The proportion of protoplasts containing nuclei was estimated in a separate experiment by bis-benzimide staining and visualization under UV light irradiation. Approximately 16% contained one nucleus and 6% contained two or more nuclei. Regeneration frequencies for protoplasts of both avenae and tritici varieties were normally between 5 and 10%. Plates were incubated at 22 C for 1-3 weeks and fast growing colonies were picked and retested on the appropriate selective media. Rates of transformation of protoplasts to phleomycin resistance can be enhanced by regenerating protoplasts for a few days before adding the antibiotic to the plates. In our hands, however, it was found that regeneration of mutated protoplasts before the addition of inhibitor resulted in high levels of background growth which interfered with selection of resistant colonies. The following mutants resistant to these inhibitors were selected:

* The figures here represent the mean from two or more experiments
1280 G. graminis var. tritici colonies surviving NQO treatment were screened for auxotrophy; 1 methionine and 1 arginine auxotroph were isolated together with a mutant requiring NH2+ as a nitrogen source. In addition, 6 color and 5 morphological mutants were isolated from the tritici isolate, and 2 color and one morphological mutant were isolated from the avenae isolate.
In conclusion, this method has provided us with an effective means of mutagenizing this organism: 195 mutants were produced using NQO mutagenesis compared with only 7 mutants reported for NTG mutagenesis (Rochefrette et al.) although differences in selection procedures and strains used may render this comparison invalid. No direct comparison of NTG and NQO mutagenesis rates was done. Work is in progress to characterize the mutants so far isolated.
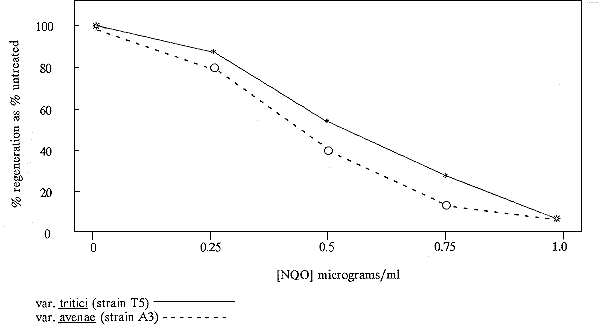
Figure 1. Protoplast survival after NQO treatment for 1 hour (Regeneration frequency is expressed as a
percentage of untreated protoplast regeneration - typically 5-10% of untreated protoplasts regenerated)