Genetic analysis of biosynthetic pathways for fungal secondary metabolites depends on availability of efficient and dependable assays for the end products. Some fungal plant pathogens produce secondary metabolites called host-specific toxins. Until recently, all bioassays for these toxins required use of whole plants or plant parts (Yoder 1981 In: Toxins in Plant Disease, Durbin ed., pp. 45-78). Since host-specific toxins, by definition, affect only plants that are susceptible to the toxin-producing fungus, other plants, animals and microorganisms are not sensitive and therefore cannot be used in bioassays. An opportunity to circumvent this problem arose when the cellular target of T-toxin (produced by Cochliobolus heterostrophus) and PM-toxin (produced by Mycosphaerella zea-maydis) was discovered to be a 13 kD polypeptide encoded by the corn mitochondrial gene Turf13. E. coli cells expressing Turf13 are sensitive to these toxins (Dewey et al. 1988 Science 239:293- 295), both of which are polyketides with similar structures and biological activities. We report here the use of toxin-sensitive E. coli cells for both qualitative and quantitative toxin bioassays.
Cells of strain DH5alpha carrying the Turf13 plasmid pATH13-T are recovered from storage in 25% glycerol at -70 C (cells can be used for no longer than 1 week after recovery from storage), streaked on an L plus ampicillin (100 ug/ml) plate, incubated overnight, restreaked from single colonies on L plus ampicillin, inoculated into 100 ml liquid core medium [M9 salts and per liter: 1 M MgSO4 (1 ml), 1 M CaCl2 (0.1 ml), casamino acids (5g)] plus ampicillin and tryptophan (20 mg/liter, filter sterilized), and incubated with shaking (250 rpm) at 30 C overnight. Cells are diluted 1:10 with core medium plus ampicillin (OD550 ~ 0.07-0.10). After 1 hr. shaking, indoleacrylic acid (0.5 ml of a 1 mg/ml stock in 100% ethanol) is added (to induce the trpE promoter which drives Turf13) and shaking is continued for 2 hr at 30 C (OD550=~0.1-0.2). Cells are spread with a glass rod on the surface of L plus ampicillin agar medium (1 ml cells/15 cm petri dish). To assay for toxin in fungal cultures, cylinders of agar medium (4 mm dia.) bearing mycelium are placed mycelium side down on the spread surface and incubated overnight at 30 C. Clear zones in the bacterial lawn surrounding cylinders indicate a positive toxin reaction (Fig. 1). More uniform distribution of bacterial cells can be obtained by embedding them in a top agar overlay (induced cells are diluted 1:1 with L plus ampicillin containing 2% agar). Either method of applying cells can be used to test for toxin in solution by adding 5 ul of solution (e.g. culture filtrate or purified toxin preparation) to a filter paper disk lying flat on the agar surface.
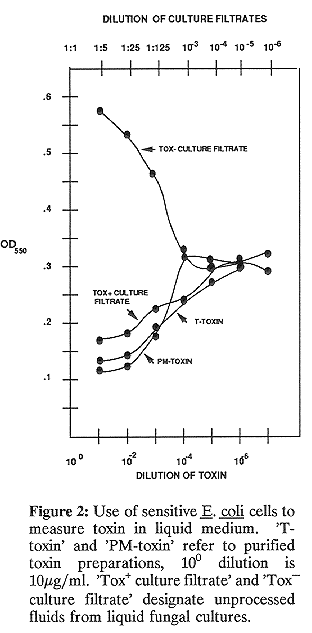
Quantitative assays can be performed in liquid medium. Toxin-containing solution is added to cells induced as described above. In a typical assay 100 ul toxin is added to 1 ml cells in a glass test tube (13 x 100 mm). The OD550 is measured, cells are incubated for various periods of time at 30 C, and an OD550 is taken at each time point. Data from a representative assay are shown in Fig. 2. Note that PM-toxin is as readily quantified as T- toxin, that as little as 1 ng toxin/ml can be measured, and that this assay is good for detecting toxin in unprocessed culture filtrates from a Tox+ strain. There is no inhibition of cell growth by culture filtrate from a Tox strain, instead growth is stimulated at high filtrate concentrations (Fig. 2).