New round spore mutations in Neurospora crassa accompanying changes in a duplication
closely linked to the R locus.
D.J. Jacobson - Department of Botany and Plant Pathology, Michigan State University, East
Lansing, Michigan 48824-1312
Various genotypes can result in round ascospores in N. crassa (Mitchell 1966
Neurospora Newsl. 10:6; Barry et al. 1972 Neurospora Newsl. 19:17). I have observed that
round ascospores can also originate in crosses where one parent is the apparent breakdown
of a partial diploid heterozygous at the heterokaryon incompatibility locus het-5. The
breakdown may remove one of the duplicated chromosome segments carrying a het-5 allele
and possibly extend past the duplication and affect the nearby Round spore locus (R).
Crosses of normal chromosome sequence with the insertional translocation
T(I->II)MD2 result in a class of progeny which duplicates the far distal portion of linkage
group IR (Figure 1). The duplication covers loci un-18 and het-5 but not R (Perkins and
Jacobson, unpublished). Mutations at R are ascus dominant; in asci from R X R+, all eight
ascospores are round. R cultures also show abnormal, peach-like vegetative morphology
that is recessive in heterokaryons. Noncoverage is inferred from the cross normal sequence
R X T(I->II)MD2 R+; the ratio of R to R+ progeny (as judged by vegetative morphology)
is 2:1 indicating that the duplication progeny, Dp(I->II)MD2, are R and the locus is outside
the duplication (Figure 1).
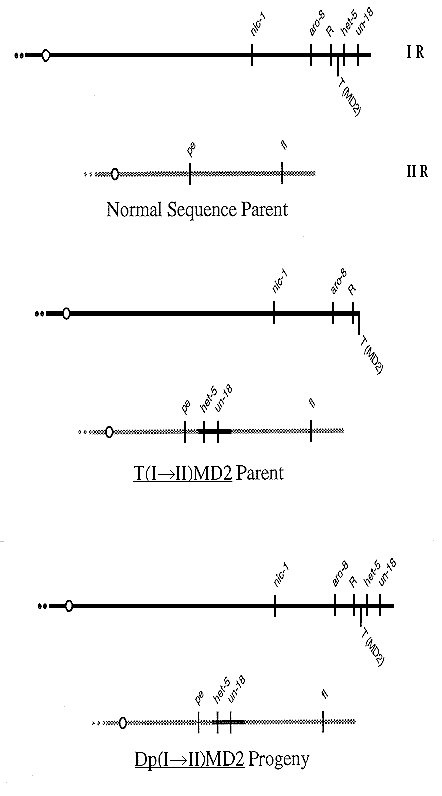
Figure 1. Partial genetic map of linkage groups I and II of parental normal sequence and T(I->II)MD2 and
Dp(I->II)MD2 progeny. het-5 and un-18 are covered, but R is not. Insertion point and orientation in linkage
group II have not been determined exactly, although translocation breakpoints have shown linkage to pe (Perkins, unpublished).
If normal sequence and T(I->II)MD2 parents in such crosses have different alleles at
het-5, Dp(I->II)MD2 progeny will be heterozygous for het-5 and will exhibit abnormal
restricted colony morphology. After prolonged incubation the colony will escape
inhibition and resume growth. (Such escape is common in duplications that are
heterozygous for a het gene and it can result from any process that eliminates the
heterozygosity, e.g. deletion or mitotic crossovers that make it homozygous.) The
morphology of the culture after escape resembles conidial separation mutants in a tap test
and is characteristic of T(I->II)MD2 and of Dp(I->II)MD2 where het-5 is homozygous. A
proportion of these escaped progeny produced round ascospores in subsequent crosses even
though R was not present in either parent.
The following set of crosses illustrates this in detail (Table 1). het-5PA was
introgressed by backcrossing from strain Panama CZ30.6 (FGSC 1311) into Oak Ridge wild
type (FGSC 2489 and 4200) which carries het-5OR. After the third backcross a het-5PA
progeny was crossed to T(I->)MD2 het-5OR. Twenty-two of 69 progeny showed restricted
colony morphology indicating heterozygous duplication of het-5. The inhibited Dp(I->II)MD2
were not stable; all escaped and grew into T(I->)MD2-like morphology. All were then
crossed to normal sequence. Only five were barren (the usual result of duplication X
normal sequence), while the other 17 were fertile to some extent as judged by production
of viable ascospores. Twelve of the fertile escaped Dp(I->II)MD2 produced only normal
shaped ascospores, three produced mixtures of normal and round, and two produced
exclusively round ascospores. Each ascus contained either all normal or all round
ascospores and each perithecium contained only one of the two types of asci. The three
crosses producing both shapes contained a mixed population of normal and round-ascospore
producing perithecia.
Progeny were analyzed from the two exclusively round ascospore crosses. No
inhibited, presumably heterokaryon incompatible, progeny were recovered. This, combined
with the T(I->)MD2-like morphology, suggests that the original Dp(I->II)MD2 lost the
het-5PA allele when it escaped and either remained a duplication or reverted to translocation
sequence. In any case, they did not produce duplications heterozygous for het-5 in
subsequent crosses to normal sequence het-5OR.
Three vegetative morphologies were seen among the second generation progeny: wild
type (14), T(I->)MD2-like (18), and a peach-like morphology which has been associated
with R (Barry et al. 1972 Neurospora Newsl. 19:17). Morphology was correlated with the
shape of the ascospores produced when these progeny were crossed to normal sequence.
If they were fertile, cultures with wild type morphology and T(I->)MD2-like cultures both
produced only normal ascospores. However, the majority of the T(I->)MD2-like cultures
were barren and this would suggest that they were newly generated duplications. The
peach-like cultures produced only round ascospores. The round ascospore trait was
heritable through another cross to normal sequence. Yet another morphological class was
apparent among the progeny of this cross. This class exhibited dense mycelium appressed
to the agar and produced exclusively round ascospores in crosses to normal sequence.
These new round ascospore strains resemble the known R mutant by being ascus
dominant, female sterile in heterozygous crosses and completely sterile in homozygous
crosses. Crosses between these strains and R are also sterile.
Table 1. Analysis of the novel round ascospore progeny originating from Dp(I->II)MD2 X normal
sequence.
Cross Number of Vegetative Ascospore
Progeny Tested* Morphology Shape
1. het-5PA X T(I->II)MD2 het-5OR 5 MD2-like --
12 MD2-like wild type
3 MD2-like wild type
2 MD2-like and round
2.& First generation Round X 14 wild type wild type
normal sequence 4 MD2-like wild type
14 MD2-like --
4 peach-like round
3.& Second generation Round X 36 wild type wild type
normal sequence 2 wild type --
3 MD2-like wild type
15 MD2-like --
1 appressed wild type
mycelium
11 appressed round
mycelium
* Only duplication progeny are listed for cross 1. All originally exhibited inhibited
morphology, but escaped after prolonged incubation. Total progeny tested listed for crosses
2 and 3.
Morphology of the MD2-like culture resembles conidial separation mutants in a tap test
and is characteristic of T(I->II)MD2 and of Dp(I->II)MD2 where het-5 is homozygous. The
morphology of the appressed mycelium class in cross 3 consisted of dense mycelium
appressed to the agar.
Ascospore shape produced in crosses of progeny X normal sequence. Wild type is spindle
shape; round is spherical similar to R mutant; (--) is barren in crosses with no ascospores
produced.
het-5PA had been backcrossed three generations to normal sequence Oak Ridge wild type
(FGSC 2489 and 4200).
& Progeny from the previous generation which produced exclusively round ascospores were
used as male parents and individually crossed to normal sequence (FGSC 2489 and 4200).
The number of progeny were pooled from all crosses.
The escaped Dp(I->II)MD2, or derived progeny, that produced round ascospores could
not be classified as either normal or parental T(I->)MD2 sequence. In each cross, white
inviable ascospores were produced in varying proportions. This was true in each of the
crosses described above and when the round ascospore strains were crossed to T(I->)MD2.
Distribution of white ascospores in unordered asci shot from perithecia was inconclusive in
determining the nature of chromosome sequence. Direct examination of rosettes also did
not reveal patterns characteristic of heterozygous T(I->)MD2 or known simple
rearrangements. Moreover, a portion of progeny in each generation were barren in
subsequent crosses. This suggests possible novel chromosome aberrations which may be
producing duplication progeny.
A proportion of escaped Dp(I->II)MD2 from a seventh backcross het-5 X T(I->)MD2
also produced round ascospores, confirming the correlation with the escape of heterozygous
het-5 duplication rather than other interactions of Panama and Oak Ridge backgrounds.
Dp(I->II)MD2 homozygous for het-5OR was stably barren, thus supporting the conclusion.
Somatic instability of duplications is common when they are placed at selective
disadvantage, for example when heterozygous for het genes (Perkins and Barry 1977 Adv.
Genet. 19:133-285). The unusual aspect of this study is that a gene outside the duplicated
area is apparently affected during breakdown of the duplication. A possible explanation is
that the distal portion of the normal linkage group IR is lost during escape resulting in
quasi-translocation sequence. This is also unusual since most studied duplication breakdowns
yield normal sequence (Perkins and Barry 1977 Adv. Genet. 19:133-285). The lost portion
of IR may occasionally extend proximally beyond the T(I->)MD2 breakpoint and thus affect
the R locus. Some Dp(I->II)MD2 which have escaped appear to be mixtures of round and
normal ascospore genotypes, suggesting that either loss of distal IR occurs repeatedly in a
colony or the chromosome sequence after deletion is itself unstable.
The round ascospore trait became stable after the first cross. It is unclear, however,
if the chromosome sequence stabilized after meiosis. The varying proportion of white
ascospores, differing colony morphologies, and barren progeny cannot be simply explained
and requires further investigation.
These observations were incidental to a study carried out at Stanford University to
localize het-5 right of the T(I->)MD2 breakpoint. Support from PHS Grant AI-01462 and
helpful suggestions from members of David D. Perkins laboratory are gratefully
acknowledged.
