Protoplast fusion techniques were used to induce parasexuality in Trichoderma reesei and the products of fusion and segregation analyzed in order to get genetic data regarding the three cellulase markers, exoglucanase (exo), endoglucanase (endo) and beta-glucosidase ( beta- glu) and their location with respect to other auxotrophic markers. Five mutants derived from T. reesei QM 9414, a hypercellulase producer, were used in this study.
Thirty-eight hour old conidia at a concentration of 1 x 10(6) spores/ml, after 1 h preincubation, were treated with 8 mg/ml Novozyme 234 for 5 h in medium containing 0.4 M (NH4)2SO4 as the osmoticum. Protoplast fusion was performed using polyethylene glycol (PEG) according to the procedures of Bos et al. (1983 Experientia Suppl. 45:298-299). Diploids were confirmed by their ability to segregate in the presence of p-fluorophenylalanine (pFPA). pFPA (7 g/ml) incorporated in complete medium (g/l: glucose, 10; yeast extract, 3; KH2PO4, 10;agar, 20) was used to induce marker segregation. The haploidizing medium was supplemented with amino acid requirements of both the parents to overcome selection against auxotrophic markers. The segregating sectors were picked randomly, purified and repeatedly subcultured on pFPA plates until stable sectors were obtained and then characterized on minimal medium (yeast extract was deleted from complete medium) supplemented with amino acid(s) as required (to a final concentration of 10 mM). Cellulase production, in complete medium broth containing 1% microcrystalline cellulose as the carbon source was measured in terms of exoglucanase, endoglucanase and beta-glucosidase activity (Sandhu and Kalra 1982 Trans. Brit. Mycol. Soc. 75:281-286). In a polygenic situation, as appears to be the case in the three cellulase components, the polygenes which represent an increase in the metric character above the mean value were taken as positive and those below it as negative.
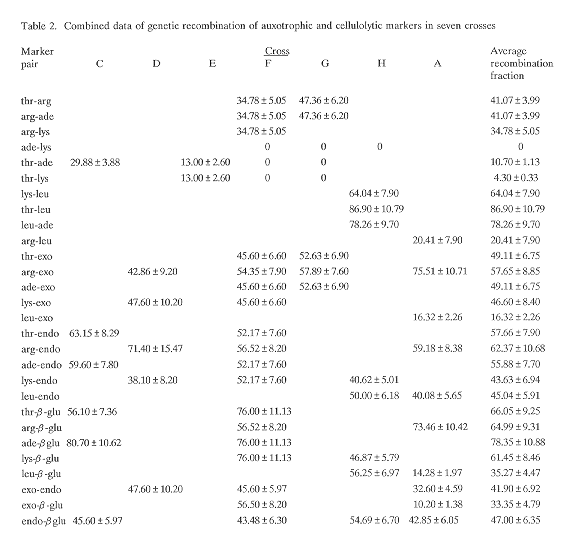
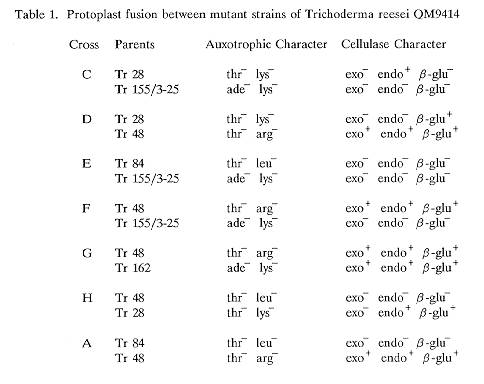
Induced heterokaryosis via protoplast fusion was used to isolate seven fusants (Table 1) with different combinations of genetic markers. Auxotrophic and cellulolytic characterization showed that the segregants of each cross were distributed into various genotypic classes, both parental and non-parental, although the allelic ratios and the independent assortment of markers, as is common in haploid parasexual progeny (Croft and Dales 1981 Proc. Int. Symp. Basel pp. 179-186) did not follow the Mendelian expectation. The average recombination fractions as calculated from the seven crosses (Table 2) were found to be low among ade-lys, thr-ade and thr-lys, indicating absence of independent segregation and linkage between them. In parasexual progeny the fact that any recombinants are formed between apparently linked markers suggests that the rare event of mitotic crossing over has taken place (Bradshaw et al. 1983 J. Gen. Microbiol. 129:2117- 2123). Low recombination fractions show that either the markers are closely linked and crossing over frequent or that they are far apart and crossing over infrequent. High recombination between markers which are linked, as in the case of thr-ade in cross C, could be the result of mitotic crossing over having occurred prior to segregation giving rise to a clone of recombinants (Debets et al. 1989 Can. J. Microbiol. 35:982-988). Other auxotrophic markers registered high recombination fractions revealing the absence of linkage between them. Recombination data of the genes responsible for the formation of cellulolytic enzymes showed their presence on separate linkage groups. In T. koningii, Wey et al. (1991 Fungal Genetics Newsl. 38:46) found gene probes of cellohydrolase and endoglucanase to hybridize to the same chromosome. As is evident in Table 2, the recombination fractions of the same marker pairs are close but not reproducible, because even slight variations in concentration of haploidizing agent are critical. One thr+ segregant was isolated from each of the two crosses made between two thr parents indicating the non-allelic nature of the mutations.
On pooling the data from the crosses studied, it can be estimated that T. reesei has at least six linkage groups with the following markers: I (thr, ade, lys); II (arg); III (leu); IV (exo); V (endo); and VI (beta-glu). Pulse field electrophoretic separation of intact T. reesei chromosomes, for which no previous karyotype is available, by Gilly and Sands (1991 Biotechnol. Letts. 13:477-482) also registered the presence of six chromosomes. The results of our studies indicate that the protoplast manipulation technique is a powerful tool for the location of markers and the identification of linkage groups in industrially important fungi like Trichoderma which lack inherent sexual processes.