Production of tyrosinase defective mutants of Neurospora crassa
Angel M. Fuentes, Ian Connerton and Stephen J. Free - AFRC, Institute of
Food Research,
Earley Gate, Whiteknights Road, Reading, RG6 2EF, UK and Department of Biological
Sciences, SUNY/Buffalo, Buffalo, NY 14260-1300
We have produced mutants defective in tyrosinase activity using the RIP procedure
(Selker et al. 1987 Cell 51:741-752, Marathe et al. 1990 Mol. Cell. Biol. 10:2638-2644).
The
tyrosinase gene has been shown to be a useful reporter in gene expression experiments
(Kothe et al. 1993 FGN 40: 43-45). The availability of tyrosinase mutants will assist in
these
studies and will also be of use for future protein engineering experiments on tyrosinase
(Fuentes et al. 1993 FGN 40:38-39).
A qa-2 arom-9 al-2 strain of N. crassa was cotransformed with the qa-2-containing
plasmid pRAL-1 (Akins and Lambowitz 1987, Cell 50:331-345) and the plasmid
GRG-1/TYR103 (Kothe et al. 1993 FGN 40:43-45), which contains the tyrosinase gene
sequences. The transformed strain was crossed with the wild-type strain 74-OR23-1A.
Mutants defective for tyrosinase activity were isolated and used as males in crosses with
am132 mutants of both mating types. Several progeny containing both mutations
(am132and
T-) were isolated and analysed by genomic Southern blotting (Fig. 1).
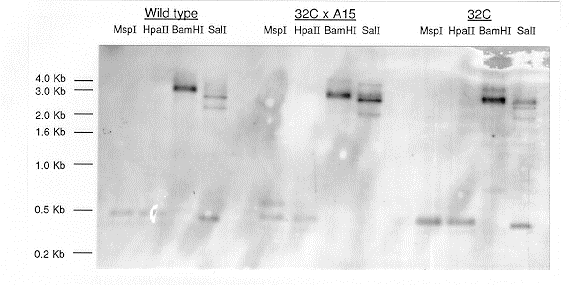
Figure 1. Southern transfers of genomic DNAs of Neurospora crassa digested with MspI,
HpaII, BamHI and SalI, probed with a 1.2 kb PCR fragment spanning the mature region of
the T gene. Wild-type (74-OR23-1A), 32CxA15 and 32C. The internal ~450 bp SalI fragment
present in the restriction digests of the wild-type and parental DNAs is noticeably missing in
32CxA15 (am132 T- al-2).
A comparison of the progeny DNA with the wild-type DNA shows that isolate 32C
(an isolate from the mating of a transformant with 74-OR23-1A) has an extra copy of the
T gene as demonstrated by the SalI and BamHI digestion patterns shown in Fig. 1. The
T
gene hybridizing sequence in isolate 32CxA15 (a T- mutant isolate from the mating of
32C
with the am mutant) has lost the ~450 bp SalI fragment. This has probably arisen due to
the
methylation of the internal cytosine residue of the SalI recognition site which prevents its
cleavage. The differential restriction pattern observed for the isochizomeric enzyme pair
MspI/HpaII in this strain also indicates the presence of cytosine methylation. These
methylation events are often accompanied by the base pair changes associated with the
RIP
phenomenon (Cambareri et al 1989 Science 244:1571-1575).
From a number of sexual crosses (>100) it is clear that all of our tyrosinase
defective
isolates are female sterile. The T- mutants are unable to complete protoperithecial
development. The isolates can, however, be used as the male partner in genetic crosses.
The
regulatory mutant ty-1 which is defective in tyrosinase induction is also female sterile.
However, unlike the ty-1 mutants which have a velvet morphology the T- mutants exhibit
a normal growth phenotype. The segregation of the tyrosinase defective phenotype can
be
followed either by directly assaying for tyrosinase activity or by following the female
sterility
phenotype.
Isolates with mutant tyrosinase genes (am132 T- al-2 and T- al-2 mutants) have been
deposited with the FGSC, which will be of value to investigators using the tyrosinase
gene
as a reporter in transformation experiments.
