a The nomenclature uses the rules of Yoder et al. (1986 Phytopathology 76:383- 385). Where allelism of the mutants has been tested, the locus is given a numeric designation.
Mutants with altered production and/or activity of certain extracellular enzymes have been obtained in Cochliobolus heterostrophus, a leaf spot pathogen of maize. The mutations should serve as useful phenotypic markers and can also be employed in studying the enzymes, their regulation, and their effect on other phenotypic traits, such as pathogenicity.
The mutants were obtained by screening survivors of mutagenesis for activity of various enzymes. Mutagenesis was done by treating protoplasts of the C-strain B30.A3.R.85 (tox1 Mat1-1) (Bronson 1988 Genome 30:12-18) with ultraviolet light at a rate to give 90- 99.9% kill (Thorson and Bronson, unpublished). Survivors were transferred to CMS (Yoder 1988 Advances in Plant Pathology 6:93-112) and then onto solid media containing various substrates to screen for enzyme activity. Production of polygalacturonase was assayed on N5 medium (Durrands and Cooper 1988 Appl. Microbiol. Biotechnol. 28:463-467) with 2% sorbose. The other enzyme detection media were CMS amended with the addition of either 4 g gelatin/l to detect protease, 4 g 4-methyl-umbelliferone-ß-D-xyloside (Sigma)/l to detect ß-D-xylosidase, 1.5 g remazol brilliant blue-xylan (Sigma)/l to detect ß-1,4-xylanase or 1 g ostazin brilliant red hydroxyethyl cellulose (Sigma)/l to detect cellulase (endo-ß-1,4- glucanase). Extracellular enzyme activity was indicated by a halo of digested substrate around a fungal colony.
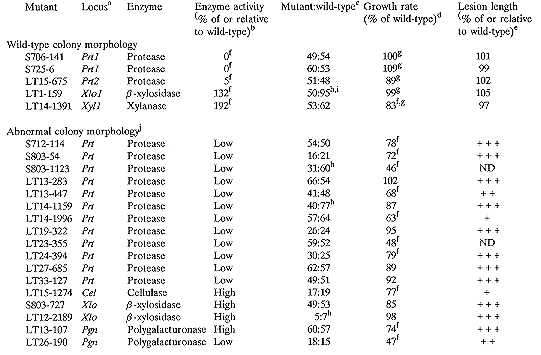
Twenty-two mutants of C. heterostrophus were obtained which consistently and heritably produce no, less, or more activity of certain extracellular enzymes than the wild- type progenitor (Table 1). Each mutant has altered activity of only a single enzyme, maintaining wild-type activity of the other four enzymes tested (data not shown). The phenotypes of the mutants segregate as single genetic loci (Table 1). Individual perithecia of crosses between the mutants and a wild-type strain contained both wild-type and mutant progeny, suggesting that the loci are nuclear. All of the mutants are prototrophic and grow vigorously, although some are distinctly different from wild-type in colony morphology and/or growth rate on solid media (Table 1). These differences in colony morphology and growth rate co-segregate with the enzyme phenotype. Differences in growth rate are probably not causing the altered enzyme activity because each mutant maintains wild-type activity of all but one of the tested enzymes.
The enzyme mutations probably comprise at least six different loci. All 115 progeny of a cross between progeny of protease mutants S706-141 and S725-6 have the parental phenotype, indicating that these two strains have mutations at the same locus. However, LT15-675 seems to have a mutated locus distinct from that in S706-141 and S725-6; in crosses between progeny of LT15-675 and the other protease mutants, 3 of 8 and 5 of 14 random progeny were wild-type, respectively, and 4 of 4 and 5 of 8 tetrads were either tetratype or non-parental ditype. Thus, two loci were identified that control protease activity. Allelism tests of the remaining mutants were not done; however, their phenotypes include altered activities of four additional enzymes, suggesting at least four more loci.
Mutant LT1-159 seems to have a regulatory defect. In the wild-type, the expression of Xlo1 appears to be regulated by xylose; on media lacking xylose the wild-type produces low ß-xylosidase activity, while on media containing xylose the wild-type produces high activity of the enzyme. However, LT1-159 produces high enzyme activity on both types of media. Thus, LT1-159 appears to have lost normal regulation and to constitutively express ß-xylosidase.
All of the mutants are pathogenic on maize, and most produce necrotic lesions similar in size to those produced by the wild-type (Table 1). Four of the mutants produce lesions distinctly smaller than wild-type; this may be attributable to reduced growth rate, which is known to reduce lesion size (Thorson and Bronson, unpublished). In addition to visual assessments of lesion size, the lesions of mutants with wild-type colony morphology and growth rate were measured to detect small differences in lesion length; none of these mutants produced lesions significantly different in length from the lesions caused by wild- type strains (Table 1). All of the mutants produced conidia from lesions when incubated at 100% relative humidity for 24 hr. Conidia isolated from lesions maintained the mutant enzyme phenotype.
The mutants are available to interested researchers upon request.
Journal Paper No. J-15981 of the Iowa Agriculture and Home Economics Experiment
Station, Ames, Iowa, Project No. 2992. Supported by grant 90-37262-5308 from the U.S.
Department of Agriculture.
Table 1. Heritability and phenotypes of mutants of C. heterostrophus with altered extracellular
enzyme activity

a The nomenclature uses the rules of Yoder et al. (1986 Phytopathology 76:383-
385). Where allelism of the mutants has been tested, the locus is given a
numeric designation.
b Enzyme activity was assessed from the diameter of haloes of digested substrate produced by uniform plugs of mycelium plus agar from two different mutant progeny of each mutant. In cases in which the diameter of the haloes was measured, the activity is given here as the average diameter of haloes produced by two progeny as a percent of B30.A3.R.85. In other cases, the diameter of haloes were visually compared to that produced by B30.A3.R.85 and are recorded here as low (40-60%) or high (120-200%) relative to wild-type. Note that the diameter of a digested halo is logarithmically related to the actual amount of enzyme activity, and so differences are probably greater than indicated here.
c The mutants were first crossed to B30.A3.R.87, a Mat1-2 sibling of B30.A3.R.85. Mutant progeny were then backcrossed to B30.A3.R.85. Crosses were done on Sach's medium and ascospores were isolated as described by Leach et al. (1982 J. Gen. Microbiol. 128:1719-1729). One spore was collected per ascus to insure that each progeny was the product of a different meiotic event. None of the segregation ratios is significantly different from 1:1.
d Growth rates of two mutant progeny of each mutant were measured on CMX (Tzeng et al. 1992 Genetics 130:81-96) after 6 days of growth at ~24°C under fluorescent lights. Except where noted, they are recorded here as the average colony diameter of two mutant progeny as a percent of B30.A3.R.85.
e Conidia from two mutant progeny were sprayed on corn plants and placed at 100% relative humidity overnight. After 4 days in the greenhouse, lesions were assessed. In cases where the lesions were measured, lengths are recorded here as average lesion size of the two progeny as a percent of wild-type. Lesion lengths of the two mutant progeny were not significantly different from wild- type, B30.A3.R.85. In other cases, lesion sizes were visually compared to those produced by wild-type (+++ = wild-type (~5-6 mm); ++ = ~3-4 mm; + = ~1-2 mm; ND = not determined because strains produce few conidia).
f Significantly different from wild-type at p=0.05.
g The average growth rate of the two mutant progeny as a percent of the average growth rate of two wild-type siblings.
h Ratios are not significantly different from either 1:3 or 1:1.
i In addition to the random progeny shown here, ten of ten tetrads tested segregated 4 mutant:4 wild-type.
j Colonies were pale, had an unusually fluffy or irregular surface, and/or produced few conidia.