
Fig. 1. Sections through red wild-type spores, showing the granular secondary wall between the mucilage (top) and the dark epispore. A, x 13800; B, x 26000.
Ascospore pigmentation mutants have been very important in studies of gene conversion and crossing-over in such fungi as Neurospora, Sordaria and Ascobolus. Most such mutants are autonomous, with each haploid ascospore's genotype controlling its phenotype. In the Pasadena strains of Ascobolus immersus, where wild-type (+) ascospores are red/brown, we have autonomous white (w) mutants at several different loci. The white spores are completely unpigmented in + x w crosses, even though the apothecia and hyphae are a mixture of + and w genotypes. The developing w ascospores are surrounded by the same ascal sap as the + spores, yet do not develop pigment even if in contact with a + spore. Possible explanations for the non-pigmentation of white ascospores are: (i) they are structurally the same as red/brown + spores but cannot make the pigment even if some precursors are present in the ascus; (ii) the mutant ascospores could make the pigment but lack some suitable structure for it to form on - a parallel would be white-eyed Drosophila of w,w;Cn,Cn;Bn,Bn genotype, which have no eye pigment because they lack the pigment-attachment protein from W, although they can make pterins and ommochrome pigments from Cn,Bn. In case (ii), the lack of a structural feature in white ascospores might change other properties, such as optimum germination conditions.
We have studied the ultrastructure and germination properties of wild-type and three white ascospore mutants in A. immersus to investigate the cause of the white phenotype and to find ways of distinguishing between different white mutants. One cannot use the cis/trans allelism test for characters expressed solely in haploid ascospores, so if two different white mutants map very close to each other, it is difficult to determine whether they are at the same locus or at two close but different loci. Double-volume spores occasionally occur in asci with fewer than eight spores, as if these large spores had two or more nuclei, but we have been unable to use them for allelism tests as they have not germinated, so their genotypes could not be checked. For allelism testing, we tried responses of ascospore pigmentation to different possible precursors of melanins. We made white mutant x same white mutant crosses of opposite mating types at 17.5 C in continuous light, with w1-10, a base-substitution at locus w1, w1-3C1, a frame-shift at the same locus, and wBHj, which is unlinked to w1. There is competition between spores for pigment precursors in Ascobolus (Lamb 1976 Microb. Gen. Bull. 40:10-12): in w62 x w62 crosses, the ascospores are pink, yet w62 spores are white in + x w62 crosses. The three mutants studied here are white in w x w and in + x w crosses. The role of melanin pigments in UV-protection and photoreactivation was studied by Lamb et al. (1992 Genet. (Life Sci. Adv.) 11:153-160). They and Helmi and Lamb (1991 Genet. Res. 57:97-103) described the general methods, strains and media used here.
For transmission electron microscopy, dehisced octads were harvested in 0.1 M phosphate buffer, pH 7.2. Spores were fixed in 2% gluteraldehyde for 4 h, then 1 h in 2% osmium tetroxide, before dehydration by 10 m in each of 50, 70, 80, 90, 100 and 100% ethanol. After two 10 m washes in propylene oxide, the spores were embedded in a hard epon-type resin (agar 100, 4.8 g, dodecyenylsuccinicadhydride 1.3 g, methylnadicadhydride 3.9 g, N-benzyldimethylamine 0.2 g), with gradual replacement of propylene oxide by the resin; pellets were hardened by 36 h at 60 C. Sections 50-90 nm thick were placed on grids and stained in 2% aqueous uranyl acetate in the dark for 30 m at 60 C. After rinsing in boiled water, grids were allowed to dry, then were placed in a drop of Reynold's lead citrate for 10 m at 20 C in a dish containing sodium hydroxide to absorb carbon dioxide. The grids were thoroughly rinsed in 0.02 M sodium hydroxide before drying.
The possible pigment precursors tried were tyrosine (up to 0.5 g/l), 3,4-dihydroxyphenylalanine (DOPA, up to 20 g/l), and catechol (up to 40 g/l) (see Bell and Wheeler 1986 Ann. Rev. Phytopath. 24:411-451 for pathways to melanins in fungi). They had no effect on pigmentation of white or red spores, so are no use for allelism tests.
In the germination experiments, the variables were plain agar versus horse dung-extract agar, and different periods (0 to 4 h) of heat-shock at 50 C before overnight incubation at 37 C. Optimum germination conditions for wild-type and all three white mutants were 2 or 3 h at 50 C on dung medium. Although there were some differences between the mutants under some conditions, the germination results did not provide reliable allelism tests.
Scanning electron microscopy showed no clear surface differences between wild-type and white ascospores. For the results of transmission electron microscopy, the terminology of Wu and Kimbrough (1992 Mycologia 83:459-466) will be used. Figure 1A shows a section of part of a red wild-type spore, with an outer layer of mucilage, a granular secondary spore wall with more electron-dense layers on the inner and especially the outer boundary, then a clear layer, an electron-dense epispore, a thick primary wall, perhaps with some outer sub-layers, then finally the spore contents. Figure 1B shows the mucilage, secondary wall, dark epispore layer, and part of the clear primary wall. Figure 2A shows an entire section of a wBHj white ascospore, and Figure 2B shows the wall structure at higher magnification. Figure 2C shows the wall structure of a w1-3C1 white ascospore from surface (uppermost) to contents. The two white ascospores completely lack the entire secondary spore wall of the wild-type spore, with the epispore being the outermost layer present, without even the transparent layer outside the epispore. Although Figure 2C, from w1-3C1, shows a narrow clear layer beneath the epispore and Figure 2A and B do not show it for wBHj, such a clear layer was not present in all w1-3C1 spores, so its presence is not a reliable allelism test.
The startling structural differences between red wild-type spores and the two white mutants' spores strongly support idea (ii), that white ascospores are white because they lack the structural layers in which the red pigment condenses in wild-type spores. This would explain why white spores are unpigmented in + x w crosses when they develop in the same ascal sap as + spores, even if in contact with + spores, when there might be diffusion of pigment precursors from + to w spores. An alternative hypothesis that the red pigments are required for condensation of the secondary wall seems less likely, as the secondary wall forms before the red pigments. The experiments with germination, pigment precursors and electron microscopy have not solved the problem of testing for allelism if morphologically identical white ascospore mutants map very close together. However, the anomaly of autonomous white ascospore mutants being expressed in + x w crosses appears to have a structural solution relating to the absence of the secondary wall in these w mutants.
Acknowledgement. We are most grateful to Ian Morris for his advice on the electron microscopy.

Fig. 1. Sections through red wild-type spores, showing the granular secondary wall
between the mucilage (top) and the dark epispore. A, x 13800; B, x 26000.
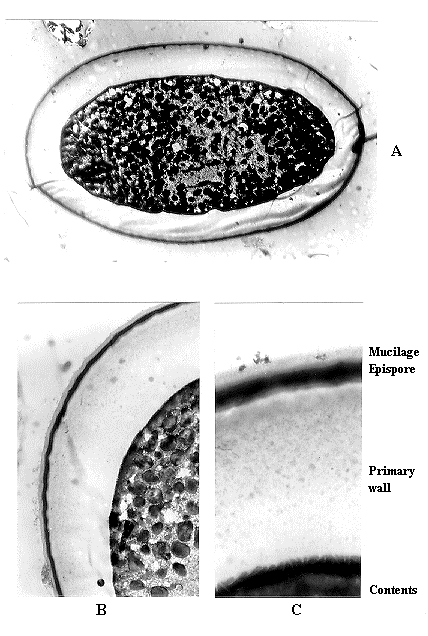
Fig. 2. Sections through white ascospores, showing the lack of a secondary wall
outside the epispore (outermost dark zone). A, wBHj, x 2900; B, wBHj,
x 6700; C, w1-3C1, x 16200.