Research was performed to determine if the anaerobic fungus, Neocallimastix frontalis EB 188, a common cellulolytic fungus of ruminants, is capable of photoreactivation and is susceptible to chemical and irradiational mutagenesis. Germination of zoospores and production of colony cellulase was measured after ultraviolet light (UVL), nitrosoguanidine or ethyl methyl sulfonate treatments. This fungus was susceptible to mutagen treatment and capable of photoreactivation after UVL exposure. Such procedures may be useful in the isolation of enhanced cellulase-producing strains.
Studies of photoreactivation and mutagen killing in filamentous fungi have been limited to aerobic species (Calza and Schroeder 1982 Mol. Gen. Genet. 185:111-119 and Schroeder 1974 Mut. Res. 24:9-16). Anaerobic fungi play a critical role in the conversion of fiber in agriculturally important ruminants (Gordon and Phillips 1993 Let. Appl. Micro. 17:220-223). Information about their ability to repair radiation damage may assist in manipulating their physiologies for enhanced fermentation. We describe here procedures which demonstrate the ability of a common anaerobic fungus, Neocallimastix frontalis EB188 (ATCC #76100), to carry out photoreactivation after UVL exposure. We also detail the susceptibility of this fungus to chemical (i.e., methyl-nitrosoguanidine, ethyl methyl sulfonate) and ultraviolet irradiation mutagenesis. Efforts to select cellulase regulatory mutants are described.
Methods
Zoospores are collected from liquid grown cultures at 70-85 hours after inoculation as previously described (Barichievich and Calza 1990 Appl. Env. Micro. 56:43-48). Culture supernatant is poured through 8 layers of sterile cheesecloth to remove rhyzoidal debris. Typically, one 300 ml round bottom flask containing 200 ml liquid culture yields 3-4 x 106 zoospores. Zoospores are spherical, motile, and average 8-10 microns in diameter and can be handled much like other spores of fungi. Exposure to air must not, however, exceed one hour since zoospore viability decreases significantly thereafter. All media and solutions used to produce zoospores contained antibiotics to control bacterial contaminants (Joblin 1981 Appl. Env. Micro. 42:1119-1122).
The ultraviolet light source was two 25-watt, low pressure germicidal lamps with a peak output of 254 nm. The fluence rate at the surface of the liquid in the exposure plates was approximately 0.02 ergs/mm2 per second. Zoospores were irradiated in 10 ml of sterile water at 10,000/ml within a plastic Petri dish (without cover). The maximum exposure time to UVL was about 10 min. All exposures were done under red safe lights at room temperature (20deg.C).
Exposure to methyl-nitrosoguanidine (MNNG) involved adding stock (dissolved in 95% ethanol v/v) to zoospores placed in either glass distilled deionized water or growth media at concentrations of 0-50 ug/ml. The amount of ethanol added to cultures had no effect on germination of zoospores. Exposure times of 30 and 60 min were tested.
Zoospores were exposed to ethyl methyl sulfonate (EMS) by placing zoospores into sterile water and adding EMS diluted with K-phosphate buffer 0.05M (pH 7) immediately before use. Exposure time did not exceed 1 hr.
To measure survival after UVL, MNNG or EMS exposure zoospores were diluted to about 100 viable zoospores per 10 ml of culture medium containing melted agar less than 46 deg C) and incubated at 39 deg C in anaerobic roll tubes for up to 3 days. Unless subsequent exposure to photoreactivation light was provided (10 min time at 39 deg C using two 40-watt cool white fluorescent bulbs, 10 cm from cultures) all roll tubes were held in opaque aluminum foil wrappers within the incubator until scored. Only obviously germinated zoospores were scored as viable zoospores (using the unaided eyes). Total counts are based on at least 600 or more colonies per treatment. All experiments were done in replicas of at least 5 each.
Screening of surviving colonies after UVL or MNNG exposure for cellulase was performed using a cellulose overlay procedure (Teather and Wood 1982 Appl. Env. Micro. 43:777-780). The solid agarose containing colonies was removed from roll tubes and melted agarose at <50deg.C containing cellulose was applied on top. The cellulose top agarose overlay contained either no glucose or 0.2% (w/v) glucose. Incubation for cellulase was at 39deg.C for up to several hours. Development of the overlay with Congo red dye (and eventually rinsing with 1.0 M NaCl) demonstrated which colonies contained rings of degraded cellulose. Tubes from each dose of the UVL and MNNG experiments were screened in a similar manner.
Results and Discussion
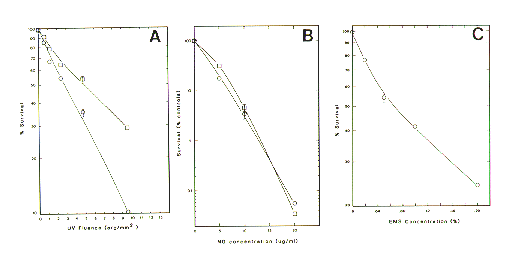
Exposure to UVL, MNNG or EMS dramatically reduces the survival of zoospores [Figure 1 A (UVL), B (MNNG) and C (EMS)]. The UVL killing curve is typical for what has been recorded in several organisms (Setlow and Setlow 1972 Annu. Rev. Biophys. Bioeng. 1:293). Exposure of zoospores to photoreactivating light (open squares versus open circles) after UVL exposure increased survival. This is what is expected of an active photoreactivation repair system. Exposure to photoreactivating light was effective if done within 1 h of UVL exposure. In experiments where photoreactivating light was provided at times 4 and 6 h after UVL exposure, no significant increase in survival was recorded (data not shown). Also, if zoospores were held at 0-4deg.C during exposure to photoreactivation light no increase in survival was recorded. Exposure of zoospores to MNNG also produced a dose-dependent killing of zoospores. An increase in exposure time from 30 to 60 min (open squares versus open circles) before plating did not apparently render MNNG more toxic. Exposure of zoospores to EMS produced a dose- dependent killing curve.
At least 4,500 colonies, isolated after UVL exposure, were scored for cellulase. One colony possessed a particularly small ring of hydrolysis around the germinated zoospore. At least 24,000 colonies obtained after exposure to MNNG were scored for cellulase. Several colonies were found to possess unusually small rings of hydrolysis. Isolation of derepressed cellulase mutants was not possible in experiments using cellulose overlays containing glucose. All putative cellulase mutant colonies failed to grow upon subsequent transfer we believe because exposure to O2 was considered excessive during the overlay procedure. Efforts are presently underway to reduce such exposure.
We conclude that the anaerobic fungus Neocallimastix frontalis EB188 is susceptible to UVL, MNNG and EMS killing in a dose-dependent manner. This fungus is also capable of photoreactivation of UVL caused damage. This is surprising since this organism is rarely in the presence of sunlight. It is not known what other (if any) DNA repair capabilities this fungus possesses. We believe it will be possible to use either UVL or MNNG (or possibly EMS) to generate and isolate negative (or depressed) cellulase mutants. Such mutants will be very helpful in our studies to elucidate the details of gene control with respect to cellulase within these fungi. These unique fungi have been shown to be essential in the normal functioning of all domestic and several wild ruminant animals (Li and Heath 1993 Can. J. Micro. 39:1003-1013) and therefore their study is important.

Figure 1. Survival of zoospores as a function of mutagen dose: (A)UVL; B(MNNG); C (EMS).
Return to the FGN43 Table of Contents
Last modified 7/25/96 KMC