Efficient Transformation of Aspergillus nidulans by
Electroporation of Germinated Conidia
Olivia Sánchez and Jesús Aguirre - Instituto de
Fisiología Celular. Uiversidad Nacional Autónoma de
Mexico. Apdo. Postal 70-242, 04510 México, D. F.,
Mexico.
We report the transformation of swollen A. nidulans conidia by
electroporation. With this method, transformation frequencies were similar to
those obtained by using protoplast fusion. The methodology employed is simple,
requiring no enzymes nor osmotic stabilizers. The effects of conidial age, DNA
topology/concentration and electric field strength are presented.
A. nidulans transformation by protoplast fusion is highly dependent on
a good enzyme-mediated protoplast preparation (Ballance et al.
1983 Biochem. Biophys. Res. Commun. 112:284-289, Tilburn et
al. 1983 Gene 26:205-221, Yelton et al. 1984 Proc.
Nat. Acad. Sci. USA 81:1470-1474). During this process, there is a
serious compromise between protoplast yield and viability, which are greatly
affected by variations in different enzyme lots, mycelial age and osmotic
medium composition.
Electroporation as an alternative transformation method has proven successful
for different types of fungi (Sánchez et al. 1993 Appl.
Environ. Microbiol. 59:2087-2092, Xoconostle-Cázares et
al. 1996 Microbiology 142:377-387, Vann, C., 1995 Fungal Genet.
Newsl. 42A:53). However, the only method reported for A.
nidulans (Richey et al. 1989 Phytopathology
79:844-847) still required protoplast production. We have adapted a
previously reported electroporation method (Sánchez et al.
1993 Appl. Environ. Microbiol. 59:2087-2092), to transform germinated
conidia at frequencies similar to those obtained by protoplast fusion.
Conidia from strain RMS011 (pabaA1, yA2, argB::trpC B, veA1, trpC801;
M. Stringer), washed 5 times with 10 ml of distilled water were used to
inoculate 400 ml of Kafer's minimal-nitrate medium plus supplements, at a
density of 1 X 107 conidia/ml and incubated at 37 C in a rotary shaker (300
rpm) for 0, 2 or 7 h. Conidia recovered by centrifugation were resuspended in
400 ml of ice-cold sterile water, centrifuged again, resuspended in 25 ml of
ice-cold pretreating buffer YED (1% yeast extract, 1% glucose) plus 12.5 mM DTT
and 20 mM HEPES (adjusted to pH 8.0 with 100 mM Tris) and incubated for 1 h at
30 C in a rotary shaker at 100 rpm. Although the experiments reported here
included DTT, we later found that it had no effect on transformation frequency.
After this 60 min incubation, conidia were centrifuged and resuspended in 2.5
ml (about 1.6 X 109 conidia/ml final) of ice-cold electroporation buffer (10 mM
Tris-HCl [pH 7.5], 270 mM sucrose, 1 mM lithium acetate) and kept on ice. For
electroporation, 1 ug of dialyzed DNA was added to 50 ul of the ice cold
conidial supension. The final volume was adjusted to 60 ul with distilled
water, the mixture was incubated on ice for 15 min and then transferred to a
0.2-cm cuvette. Electroporation was performed using the Bio-Rad Gene Pulser
and Pulse Controller Apparatus. Voltage was adjusted to 1,000 V, capacitance
to 25 uF and resistance was 400 Ohms (pulse length varied between 5.1 and 5.8 ms).
Under these conditions, about 35 % of the conidia were killed.
Following electroporation, 1 ml of ice-cold YED was added to the cuvette and
the cell suspension was transferred to a sterile 10 ml tube, kept on ice for 15
min and incubated at 30 C for 90 min in a rotary shaker at 100 rpm. Conidia
subjected to electroporation were spread on supplemented minimal plates lacking
arginine (250 ul/plate) and incubated at 37 C. Most of the plated conidia
germinated on selective medium but failed to grow further; the actual
transformants being evident after 48 h. Transformant stability was tested by
velvet-replica plating in selective medium. Only healthy growing, well
sporulated colonies were counted as transformants.
Table 1. Effect of conidial germination time and freezing-thawing on
transformation frequency by electroporationa.
Number of Transformants / ug DNA
_______________________________________________
Plasmid 0 h 2 h 7 h 2h thawed *
_________________________________________________________
None 0 0 0 0
pDHG25 30 551 97 372
_________________________________________________________
aConidia were germinated for the indicated times and electroporated, using
1 ug of the autonomous replicating plasmid pDHG25.
* 250 ul aliquots of 2 h germinated electrocompetent conidia were transferred
to -70 C, stored for several days, thawed by incubating on ice and
electroporated. Salt traces in DNA were removed by spin-filtration trough
water-equilibrated Sephadex-G25-80 minicolumns. Except for 0 h, numbers
represent mean values from two independent experiments, with a maximum
variation of 10 % about the mean.
Table 2. Effect of DNA topology and replication type on transformation
frequency by electroporation a.
Type of DNA No. of Transformants/ ug DNA
_______________________________________________________________________
pDHG25, circular 623
pDHG25, linear (BamHI) 1,189
pREN2, circular 11
pREN2, linear (KpnI) 19
_______________________________________________________________________
a DNAs digested with the indicated enzymes were filtered through Sephadex and
stored at 4 C (Navarro et al. 1996 Curr. Genet.
29:352-359). For pDHG25, numbers represent mean values from four
independent experiments. For pREN2, numbers are mean values from duplicates.
Maximum variations were 9 and 26 % about the mean, respectively.
Using this protocol, and the autonomous replicating plasmid pDHG25, which
carries the argB gene as a selective marker (Gems et al.
1991 Gene 98:61-67), we evaluated the effect of germination time on
transformation frequency. Results in Table 1 show that in the 2 h germination
time the number of transformants per microgram of DNA was about 18 and 5 times
more than those obtained after 0 and 7 h germination times, respectively. Only
2 h germinated conidia were used in all further experiments. The number of
transformants obtained with pDHG25 using the 2 h germinated conidia is slightly
higher than what we routinely obtain using protoplast fusion; 400-500
transformants/ ug DNA.
When frozen electrocompetent conidia were electroporated with pDHG25,
transformation frequency was about 30 % lower than with unfrozen conidia (Table
1). The convenience of having frozen stocks from different strains ready to be
transformed could compensate for this reduction. We used frozen conidia to
test the effects of plasmid concentration and field strength parameters on
transformation efficiency. Within the DNA range tested (100 to 2,000 ng) we
observed a non-linear response and a plateau in the number of transformants
after 500 ng of DNA (Figure 1). With Regards to the effect of field strength,
we found that 5 kV/cm (1000 Volts/0.2 cm) resulted in the highest number of
transformants (Figure 2).
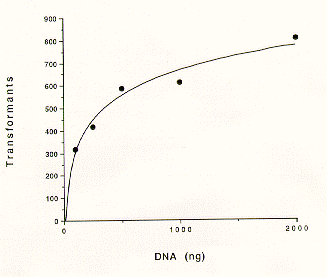
Figure 1. Effect of plasmid concentration on transformation efficiency.
Frozen electrocompetent conidia were thawed, incubated with the indicated
amounts of circular pDHG25 and electroporated. The results are means from
duplicates with a maximum variation of 4 % about the mean.
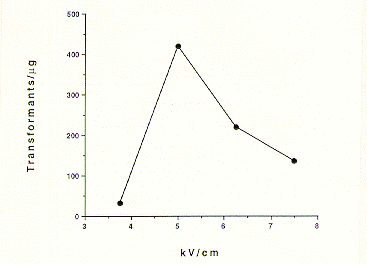
Figure 2. Effect of Field Strength on Transformation Frequency.50 ul
aliquots from a frozen conidial pool were incubated with 1 ug of circular
pDHG25 and electroporated using the indicated kV/cm.
DNAs with different topology and replication types were also tested for
electroporation efficiency. Results in Table 2 show that transformation
frequency for the integrative plasmid pREN2 was much lower than for the self
replicating plasmid pDHG25. However, the number of transformants obtained with
pREN2 is also similar to those obtained by us and others when using protoplast
fusion (5-10 transformants/ ug; Upshall, A. 1986 Curr. Genet.
10:593-599). Using linear forms of both types of plasmids increased
transformation frequency about 2 times, perhaps reflecting an increased
frequency of DNA entrance to the cells. Southern blot analysis of several
transformants using argB and catA as probes showed that 8 out of
8 PDHG25-derived transformants contained argB sequences as part of the
self replicating plasmid, whereas from 9 pREN2-derived transformants, 1
contained pREN2 sequences integrated at catA , 7 contained a single copy
of pREN2 integrated at other genomic regions and 1 contained multiple
integrations. Although we have not tested other electroporation apparatus,
conditions described here are not too different from those reported for yeast
(Becker and Guarente 1991 Methods in Enzymology 194:182-187).
Therefore, yeast protocols for other electroporators should be a good starting
point, provided the use of germinated conidia and a self replicating plasmid to
optimize conditions.
ACKNOWLEDGMENTS
Supported by grants No. N9109-0708 from CONACyT and IN200192 from DGAPA-UNAM,
Mexico. We want to thank Alfredo Martinez for valuable comments and
suggestions, J. Clutterbuck for plasmid pDHG25, W. Hansberg for critical
reading and Ch. Staben, M. Katz, P. Margolis, D. Vann and D. Eastwood for
comments via bionet.mycology.
Return to the FGN43 Table of Contents
Return to the FGSC Home page

Contact the FGSC
Last modified 7/25/96 KMC


