S. S. Sandhu1 and G. Venkateswerlu2 - 1Dept. of Biological Science, R.D.University, Jabalpur, 2Dept. of Biochemistry, Osmania University, Hyderabad, India
Generally niaD mutants of fungi are selected by spontaneous mutations on appropriate minimal medium supplemented with various concentrations of KClO3 and a nitrogen source (Daboussi et al. 1989 Curr. Genet.15:453-456; Johnstone et al. 1990 Gene 90:181-192; Malardier et al.1989 Gene 78:147-156; Unkles et al.1989 Gene 78:157-166). But in case of entomopathogenic fungi it has been observed that niaDmutants isolated simply by spontaneous mutation on chlorate were not stable, (Table-1). Therefore a method has been developed to isolate stable niaD mutants of these fungi by treating protoplasts with ethane methane sulfonate (EMS).
The method reported here is advantageous as it yielded more mutants when compared to the method of spontaneous mutation. Secondly, mutants obtained by EMS treatment were more stable than spontaneous niaD mutants. Their reversion frequency was less than one in 107 viable conidia, whereas comparatively high reversion frequency was obtained, 100 and 60 in 107 viable conidia of spontaneous mutants of Metarhizium anisopliae and Beauveria bassiana, respectively. Protoplasts of B. bassiana and M. anisopliae obtained by the method of Shimizu, 1986 J. Seric. Sci. Japan 5:510- 517,(approximately 106) were incubated with 15 ul ethane methane sulfonate (d=1.17g, Sigma) in 1 ml of stablizing medium (0.02M phosphate buffer pH 7.2 containing 0.6M KCl and 2mM MgCl2) at 28 +/- 1 C for 1 h. These protoplasts were then washed twice with 10 ml of stablizing medium by centrifuging at 1,000 X g for 10 minutes, diluted and then spread on plates containing a regeneration medium (2% sucrose; 1% peptone; 0.5% NaCl; 3% yeast extract and 2% agar agar, pH 7.0). Conidia from surviving colonies were harvested and suspended in Tween 80 aqueous suspension (50 ul/100 ml distilled water). Dilutions of this suspension were plated again on a minimal medium (1% glucose; 0.1% K2HPO4; 0.5% MgSO4; 0.5% KCl; 0.001% FeSO4. 7H2O; 0.005% EDTA disodium salt, pH 6.5) containing 10 mM glutamate as sole source of nitrogen and 470 mM Chlorate. These plates were incubated at 28 C +/-1 C for five days.
It was found that mutants arose at a frequency of 10 and 8 in 106 viable conidia of B. bassiana and M. anisopliae respectively. These mutants were isolated and purified further on a minimal medium containing chlorate and glutamate. The ability of these mutants to grow on nitrate, nitrite, ammonium, hypoxanthin, proline and glutamate as sole source of nitrogen was assessed (Table 2). Of the 18 mutants tested, two each of B. bassiana and M. anisopliae had phenotypes indicative of niaD-. Two were found to be crnA- of M. anisopliae while one crnA- of B. bassiana was noticed. It was found on testing two niaD mutants
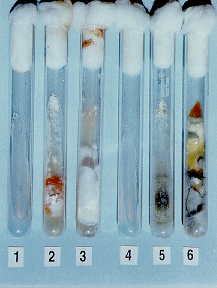
(Figure 1) designated niaD-1 and niaD-4 of B. bassiana and M. anisopliae respectively, that reversion to nitrate prototrophy was less than one in 10,sup>7 viable conidia, whereas reversion frequencies of spontaneous mutants was more than 100 and 60 in 107 viable conidia of B.bassiana and M. anisopliae, respectively. Hence EMS treated niaD- mutants should be suitable for future experiments involving protoplast fusion and transformation.
We gratefully acknowledge the financial support by DBT and CSIR, New Delhi in the form of Biotechnology National Associateship and research project to SSS, and the generous gift of Novozyme-234 from Dr.J.R.Kinghorn, Plant Science Laboratory, St.Andrews University, Scotland.
Table 1. Reversion frequencies of mutants of B. bassiana and M. anisopliae to wild type growth.a
Fungus Number of mutants isolated Reversion frequencies
Spontaneousb EMS Spontaneous EMS
B. bassiana 4 10 >100 <1
M. anisopliae 2 8 >60 <1
aReversion frequencies in 106 viable conidia.bMethod of Malairdair et al. 1989 (Gene 78:147-156) was followed.
Table 2. Properties of B. bassiana and M. anisopliae mutants defective in nitrate assimilation.
Genea Chlorate Utilization of sole nitrogen sourcec Summaryd
resistanceb A B C D E F
niaD- R - + + + + + Nitrate
Reductase
structural gene
crnA- R + + + + + + encodes nitrate
uptake in young
cells
aThe minus superscript denotes loss of functional mutations.

Figure 1. niaD-mutants of Beauveria bassiana (# 1-3) and Metarhizium anisopliae (# 4-6).#1 & 4: niaD-mutants not growing on minimal medium with nitrate as sole source of nitrogen; #2 & 5: growth on minimal medium containing chlorate and glutamate; #3 & 6: growth on complete medium.
Return to the FGN 44 Table of Contents