Use of flow-cytometry to distinguish between haploid and diploid strains of Aspergillus fumigatus
J. Ramón De Lucas, Ana I. Domínguez, Alfonso Mendoza* and Fernando Laborda - Dept. Microbiología & Parasitología. Fac. Farmacia. Universidad Alcalá de Henares Campus Universitario. 28871 Alcalá de Henares (Madrid) Spain.
* Centro de Investigación Básica. GlaxoWellcome S.A. 28760 Tres Cantos (Madrid) Spain.
Many filamentous fungi with general interest such as plant/human pathogens and enzyme/antibiotic producers lack a sexual cycle. Since sexual crosses are unavailable in these species, the parasexual analysis, apart from physical mapping, is the only way of mapping the chromosomes. The use of the parasexual cycle requires a method to distinguish between haploids and diploids. Here, we report the use of flow-cytometry to distinguish clearly between haploid and diploid strains of Aspergillus fumigatus, a very rapid, simple and accurate technique that can be applied to parasexual analysis in other filamentous fungi.
In the well known Aspergillus nidulans this can be easily accomplished when strains carrying diploid recognition systems are used (Käfer et al. 1976 Mutat. Res. 38:141-146). However, in other filamentous fungi lacking these recognition systems, such as the pathogenic fungus Aspergillus fumigatus, the major criterion for distinguishing ploidy is the spore diameter. Diploid strains have larger asexual spores because of the increase in nuclear volume.
We have found it extremely difficult to distinguish between the diameter of spores of diploid and haploid strains in A. fumigatus, especially since differences among diameters of haploid or diploid spores are commonly observed by microscopy. In addition, the method devised by Upshall et al. (1977) (J. Gen. Microbiol. 100:413-418) to distinguish between haploid and diploid strains of A. nidulans and A. terreus was unsuitable to differentiate such strains in A. fumigatus. Diploid strains of this fungus were not much more sensitive to benlate or benomyl than haploids, possibly because of the high growth rate of A. fumigatus compared with other Aspergillus species.
Flow cytometry is a powerful technique that allows measurement of functional and structural parameters such as enzyme activities, redox rate, protein and DNA content, DNA synthesis, DNA base ratio or cell size and shape in every single cell of a population (Shappiro 1993 Ann. NY Acad. Sci. 677: 155-166). In addition, the amount of data analyzed is very high (300-1000 events/sec) therefore, statistically significant determinations on large samples are easily obtained in a few minutes.
Here we report the use of flow-cytometry to distinguish clearly between haploid and diploid strains of A. fumigatus by their differences in DNA content. Additionally, we confirmed the suitability of this method by distinguishing haploid and diploid strains in several fungi including the filamentous fungus A. nidulans and the yeast Saccharomyces cerevisiae.
A. fumigatus sC and niaD mutant strains, derived from the parental strain A. fumigatus 48238 (GlaxoWellcome, Spain), were obtained by using the methods described for A. nidulans (Arst 1968 Nature 219: 268-270 and Cove 1976 Mol. Gen. Genet. 146:147-159, respectively). Heterokaryons and diploids from both strains were obtained by applying the methods already described (Stromnaes and Garber 1963 Genetics 48:653-662).
A. nidulans R21 (pabaA1 yA2) and R153 (wA3; pyroA4) (Armitt et al. 1976 J. Gen. Microbiol. 92:263-282) were used to establish heterokaryons and somatic diploids using the methods already described (Pontecorvo et al. 1953 Adv. Genet. 5:141-238; Clutterbuck 1974 Handbook of Genetics vol 1, Plenum Press, NY pp 447-510). S. cerevisiae YPH500 (haploid strain) and YP501 (diploid strain) (Yeast Stock Center) were also used in this work.
Haploid or diploid (108) conidia were resuspended in 5 ml of Tween 80 (Aldrich) saline (Armitt et al. 1976 J. Gen. Microbiol. 92:263-282) and fixed in 70% ethanol for 20 min at 4oC. Conidia were collected by centrifugation at 3000 rpm for 5 min and washed twice with Tween saline. Conidia were resuspended in TE (10 mM Tris HCl, 1 mM EDTA, pH 8) buffer, incubated at 37oC in the presence of RNAse (1 mg/ml) for 1 h and washed twice with Tween saline. DNA staining in fixed cells was carried out by adding propidium iodide (25 µg/ml) to the conidial suspension in TE buffer for 30 min. Flow cytometry analyses were carried out in a FACScan flow cytometer (Becton-Dickinson, San Jose, CA).
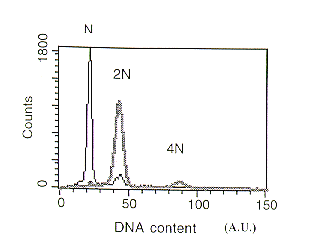
When a conidial suspension (>103 spores) of the A. fumigatus sC or niaD strain stained with propidium iodide was applied to the flow-cytometer, a peak of fluorescence (22 arbitrary units (AU)) corresponding to the haploid (N) content of spores was obtained. When we measured the DNA content of a spore suspension of the A. fumigatus sC::niaD diploid (2N) strain a fluorescence value of double that for the haploid strain was observed (Figure 1) reflecting the increased DNA content.
Using the same technique (flow cytometry) we were also able to distinguish between haploid and diploid spores of A. nidulans (Figure 2) by monitoring the value of the fluorescence peak obtained. Haploid spores gave a major peak of 24.7 AU, while diploid (2N) conidia showed a peak of fluorescence at approximately two-fold higher value (43.2 AU). Similar results were found when haploid and diploid cells of the yeast S.cerevisiae were employed (data not shown), although the fluorescence value (AU) for the (N) or (2N) DNA content was lower than those observed in Aspergillus as result of their lower amount of total DNA per cell.
Additionally, a small peak (around 10% of total fluorescence) was observed in preparations of haploid and diploid spores of A. fumigatus and A. nidulans. The DNA content of this minor peak corresponded to the double of that recorded for the main peak. It seems likely that this peak reveals the presence of a minor population (around 10% of total) of spores containing double amount of DNA than that observed in the bulk of a conidia suspension. As control, we analyzed the content of DNA in haploid and diploid cells of S. cerevisiae arrested at the stationary phase. In these samples, only one population of cells with respect to the DNA content (N and 2N for the haploid and diploid, respectively) was observed.
It is possible that this low population of A. nidulans and A. fumigatus spores could have arrested after reaching the S-phase before dormancy. Bergen and Morris (1983) (J. Bacteriol. 156:155-160) demonstrated that dormant conidia of A. nidulans are arrested at some stage before completion of S-phase since germination of conidia yielded uninucleate cells in the presence of hydroxyurea. By determining the time that hydroxyurea treated spores required to complete the cell cycle (undergo mitosis phase), these authors concluded that dormant conidia are arrested at, or before, the start of S-phase. Results in our work, by measuring the DNA content of dormant conidia, suggest that a sub-population of dormant spores (about 10% of haploid and diploid conidia) of A. nidulans and A. fumigatus could contain double the amount of DNA probably due to their arrest after duplication of DNA. In addition, another possibility, such as this 10% subpopulation could include binucleate spores cannot be ruled out until ultrastructural studies are carried out.
This work demonstrates that the use of flow cytometry is a very rapid, simple and
accurate method to distinguish diploid and haploid spores in Aspergillus species. This technique
could also be applied to parasexual analysis in other filamentous fungi, especially in those
species of industrial interest where sexual crosses are unavailable (i.e., Penicillium chrysogenum,
A. niger, A. oryzae, A. awamori...).

Figure 1. DNA content of Aspergillus fumigatus conidia analyzed by flow cytometry. Thin black
line :Haploid strain. Broad grey line: Diploid strain (N) DNA content (22A.U.).
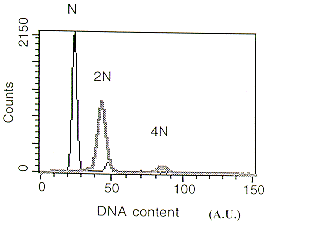
Figure 2. DNA content of Aspergillus nidulans conidia analyzed by flow cytometry. Thin black line :Haploid strain. Broad grey line: Diploid strain (N) DNA content (25A.U.).