Germination and plating efficiency of Neurospora crassa microconidia
R. Kalpana, K. K. Adhvaryu and R. Maheshwari Department of Biochemistry, Indian Institute of Science, Bangalore 560012, India.
The plating efficiency of microconidia , estimated by the number of colonies formed on a sorbose-containing medium, is commonly taken as a measure of their germinability. It has generally been low. We report that when microconidia of mcm and pe; fl strains were spread on a dialysis membrane overlying sorbose medium, their germination was greater than 80%.
The experiment was repeated with pe; fl microconidia whose plating efficiency has ranged from 1 to 95% (Munkres 1977 Neurospora Newsl. 24:9-10), although a value of 7-30% was reported by other investigators (Barratt 1964 Neurospora Newsl. 6: 6-7; Maheshwari 1991 Exp. Mycol. 15: 346-350). In the present experiments, the plating efficiency of microconidia from 7day-old plate cultures of a pe; fl strain (FGSC #3072) was 14 40%. After 18 h on dialysis membrane, 40% of microconidia were swollen and 25% had formed germ tubes (Figure 2). After 24 h, ungerminated microconidia per microscope field were very few; 80-90% microconidia had produced hyphae. We also tested germination of fl;dn (FGSC #3517) microconidia which gave a plating efficiency of only 2% on sorbose medium. By contrast, after 48 h, 40% microconidia had germinated on overlaid dialysis membrane.
The results indicate that microconidia of some N. crassa genotypes are capable of high germination frequencies, comparable to that of macroconidia. Despite their high viability, the plating efficiency of macroconidia and microconidia has differed significantly. The low plating efficiency of microconidia might be because of an inhibitory effect of some substance that is commonly present in the medium but which is not diffusible through dialysis membrane. Alternatively, contact stimulation by dialysis membrane may improve the germination of microconidia. The plating efficiency of mcm microconidia (test strain) was not improved when Difco Noble agar or agarose was used. Substitution of sorbose by tergitol for inducing colonial growth (Springer 1991 Fungal Genetics Newsl. 38: 92) resulted in no colonies being formed. This indicated that microconidia and macroconidia are differentially sensitive to tergitol or some other component of the sorbose plating medium. A future research objective is to translate the high viability of microconidia into a practical method of obtaining high plating efficiency.
Acknowledgment: This research was supported by Department of Biotechnology, Government of India.

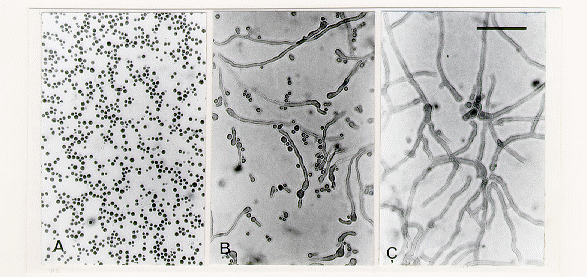
Figure 1. Bright-field microscopy of mcm microconidia stained with cotton blue. All micrographs are at same magnification. Scale bar = 50 µm. (A) Appearance of microconidia before spreading on membrane. (B) Swelling and germination of microconidia after 6 h on dialysis membrane overlying sorbose medium. (C) After 15 h.
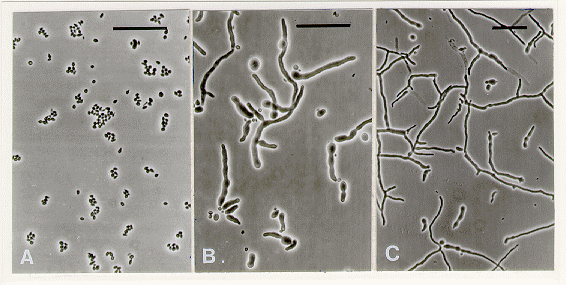
Figure 2. Phase contrast microscopy of pe; fl microconidia. Scale bar = 50 µm. (A) At 0 h on dialysis membrane spread over sorbose medium. (B) At 12 h. Some microconidia have swollen and formed fat germ tubes. (C) At 18 h. Some microconidia have produced branched germ tubes.