Isotope discrimination by the stearoyl desaturase of Neurospora
crassa.
FGN46:9-10
Marta Goodrich-Tanrikulu, Allan E. Stafford, and Thomas A. McKeon - U. S. Department of Agriculture, Agricultural Research Service, Western Regional Research Center, Albany, CA 94710, U. S. A.
In wild type Neurospora crassa, we previously observed an unexpectedly low proportion of label in unsaturated fatty acids that should have been derived from deuterated palmitate (16:0). Interpretation of whether the position of deuteration could account for these results was inconclusive, because wild type had a low proportion of total fatty acids derived from the deuterated supplement. The N. crassa cel-1 strain is ideally suited for studies of fatty acid enrichment, because cel-1 must obtain its fatty acids exogenously. We supplemented cel-1 with 16:0's deuterated at different positions. With each of the labelled 16:0's, cel-1 desaturated the stearate (18:0) derived from the 16:0 more efficiently than wild type. However, when the 16:0 was deuterated at the site of future desaturation, we indeed observed effects on fatty acid composition and label distribution consistent with inhibition of the 18:0 desaturase.
In order to magnify potential effects of deuteration on 16:0 metabolism, we compared the metabolism of these 16:0's deuterated at different positions in the N. crassa cel-1 (FGSC #819) mutant. This mutant (formerly cel) has an impaired fatty acid synthase and must obtain virtually all of its fatty acid from added supplements. (Because its lipids can consequently be enriched with the fatty acids that are used as supplements, cel-1 has been extensively used to study how fatty acid composition affects membrane properties and physiological processes.) The cel-1 mutant grows most efficiently if it is supplemented with 16:0, and it must elongate and desaturate the 16:0 to other fatty acids. We reported that the proportion of deuterated fatty acids is far higher in cel-1 supplemented with [7,7,8,8-2H4]16:0 than in wild type, and that cel-1 desaturates the 16:0 more efficiently (Stafford et al. 1998 Lipids 33:303-306). In that report, we also mentioned having obtained evidence for an isotope effect on 18:0 desaturation, which we present here.
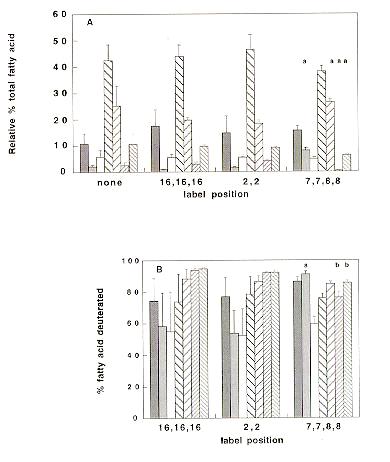
Whereas the fatty acid compositions of cultures supplemented with [2,2-2H2]16:0, [16,16,16-2H3]16:0, or undeuterated 16:0 were not significantly different, cultures supplemented with [7,7,8,8-2H4]16:0 had an approximately 5-fold greater proportion of 18:0 relative to total fatty acids (Figure 1A). They also had a lower (8-fold) proportion of 20:2, as well as a lower proportion of 20:3 (these two fatty acids are unique to cel strains).
The difference in fatty acid composition of cultures supplemented with [7,7,8,8-2H4]16:0 suggests that once the supplement has been elongated to [9,9,10,10-2H4]18:0, it is not as efficiently utilized as a substrate for conversion to 18:1, compared to 18:0 not deuterated at these positions. Once 18:1 has been formed, it is capable of being desaturated via 18:2 to 18:3, and further elongated. The reduced elongation of 18:2 and 18:3 to 20:2 and 20:3 in cultures supplemented with [7,7,8,8-2H4]16:0 probably results from the decreased availability of 18:1.
The proportion of each fatty acid deuterated in cultures supplemented with the three 16:0's also indicates differences in their metabolism (Figure 1B). For cultures supplemented with [2,2-2H2]16:0 or [16,16,16-2H3]16:0, the largest decrease in proportion of deuteration was between 16:0 and 18:0, suggesting that elongation of the 16:0 to 18:0 is a rate-limiting step in these cultures. In contrast, cultures supplemented with [7,7,8,8-2H4]16:0 show the largest decrease between 18:0 and 18:1, suggesting that the desaturation step is rate-limiting. Our evidence is consistent with deuteration at the 9 or 10 position inhibiting the 18:0 desaturation reaction. These findings in N. crassa are consistent with those in the yeast Saccharomyces cerevisiae, which demonstrated inhibition of desaturation by that isomer of a fatty acid analog deuterated at the 9 position (Buist and Behrouzian 1996 J. Am. Chem. Soc. 118:6295-6296). Similarly, deuteration or tritiation affected desaturation at the 9 position by the desaturase of the bacterium Corynebacterium diphtheriae (Schroepfer and Block 1965 J. Biol. Chem. 240:54-63). All of these observations are indicative of a primary kinetic isotope effect, with C-H bond cleavage at the 9 position being the rate-limiting step in the desaturation reaction.
In S. cerevisiae and C. diphtheriae, 18:1 is the final product of
the desaturation pathway, whereas N. crassa synthesizes additional unsaturates.
Inhibition of the 18:0 desaturase in cel-1 has measurable in vivo effects
on these further desaturation and elongation products, effects that have not been previously
demonstrated in other organisms. Our results demonstrate changes in fatty acid composition
attributable to isotope discrimination by the N. crassa stearoyl desaturase.

Figure 1. 16:0 metabolism by the N. crassa cel-1 mutant. A) Relative percent total fatty acid composition of cultures supplemented with undeuterated 16:0, or 16:0 with deuterium present at various positions of the fatty acid chain. B) Percentage of each fatty acid that was deuterated, determined by GC/MS with selective ion monitoring.
Fatty acid key:
Values are averages ± SE for 3 experiments (6 for [7,7,8,8-2H4]16:0). a, P < 0.01, and b, P < 0.05 that averages do not differ from the other deuterated 16:0's (Student's t test).
Return to the FGN 46 Table of Contents