A heterokaryon instability gene in the Rockefeller-Lindegren strains of Neurospora crassa and its possible relation to the het i gene in Oak Ridge-St. Lawrence strains
J.F.Wilson1, Charles P. Calligan (deceased)1, and Jo Ann
Dempsey2, 1Biology Department, Univ. of North Carolina at
Greensboro, Greensboro N, 2 Dept. Of Microbiology and Immunology, School of
Medicine, Univ. N. Carolina at Chapel Hill, Chapel Hill NC
Fungal Genetics Newsletter 46:25-30
The het I/i genes in the Oak Ridge-St. Lawrence (OR-SL) strains of Neurospora crassa are unique among the het genes in that they do not cause cell death in incompatible heterokaryons. Instead, within certain nuclear ratios, forced heterokaryons are unstable. They become homokaryotic for the het I component, and stop growing. Similar alleles have now been found in Rockefeller-Lindegren (RL) strains. The latter differ from those of the OR-SL strains in that the heterokaryons become homokaryotic over a wide range of initial nuclear ratios. Evidence is presented that suggests the two sets of alleles may be the same.
Stage II has been studied extensively (Perkins 1988, Fungal Genet. Newsl. 35:44-46). At this stage, the mixing of protoplasts results in an incompatibility reaction which kills the fused hyphal segments if the two components differ in one or more of the het genes, C/c, D/d, or E/e. Most of the strains used today are in the Oak Ridge-St. Lawrence (OR-SL), or the Rockefeller-Lindegren (RL) genetic background. OR-SL strains are het Cde i and RL strains are het CDE (I/i genotype is unknown). There are five additional het loci identified by inhibited growth of partial duplications. Two of these also test positive for incompatibility reactions like those of het C, D, E; the rest have not been tested (Perkins, et al. 1993 Fungal Genet. Newsl. 40:69-73).
This communication presents evidence of the existence of a stage III instability variant in RL strains (provisionally called HI/hi for "heterokaryon instability"), that is similar to the I/i strains in OR-SL (Pittenger and Brawner), and some indications that the two may be at the same locus. An accompanying paper describes the first stage I (hyphal fusion) mutant to be reported.
The strains used are inl(37401), pan-1(5531), al-2(15300), nic-3 (Y31881), HC23-4A, and HC23-8a. The first four mutants are derivatives of original Beadle/Tatum mutants and do not correspond to the FGSC catalog listings. The last two are St. Lawrence wild types derived from a cross made between two of twenty isolates sent to us by Dr. St. Lawrence in 1961. The twenty isolates in turn were from a sib cross after a series of nine backcrosses to ST74A made by Dr. St. Lawrence. These two isolates, unaccountably, were het CDe instead of the standard het Cde, as were half of the remaining eighteen isolates.
The media, crossing methods, extracts, and growth measurements were Neurospora crassa techniques used in the Tatum Laboratory (Wilson and Garnjobst 1966 Genetics 53:621-631). The microsurgical methods are described in Wilson 1963 Am. J. Bot. 50:780-786. Nuclear ratios were adjusted to 1:1 with a modification of the methods used by Pittenger and Brawner. Extreme nuclear ratios were achieved by microinjection.
The test sequence used in identifying HI/hi or I/i is crucial, and should be emphasized. After nutritional and sex tests, we identified the inl; het CDE isolates by inoculating minimal slants with preconidial aerial hyphae of the isolates and pan; al-2; CDE and CDe testers. A fully compatible het C,D,E combination will cover the surface of a 16-18 mm minimal slant in 24 h at 30C. The inl; het CDE isolates were then tested for HI/hi genotype by forming heterokaryons on minimal slants with pan-1; al-2; CDE HI and hi testers. Mixed hyphal-conidial inocula were used at this step. All tests were incubated at 30C, never higher, to avoid what we now know as the scot phenotype in RL strains (Perkins and Bjorkman 1978 Neurospora Newsl. 25:24-25). Stable and unstable combinations generally grew equally well initially, but the unstable combinations grew poorly or not at all on transfer. This procedure gives clear-cut results with het C,D,E strains, and in all but a few het C,d,e strains tested.
Most of the data reported here are from Mr. Calligan's Master's Thesis, 1976. Univ. N. Carolina at Greensboro.
This research started with the discovery that two of our cultures, both inl (37401)CDE a, behaved differently in growth tubes as forced heterokaryons with the same pan-1 (5531); al-2 (15300)CDE a. One grew normally to the end of the tube; the other stopped after 36-72 hours.
Tester strains (het CDE; HI and hi) with the above markers and nic-3 (Y31881) were obtained in both mating types, and heterokaryons were formed with progeny from crosses as indicated above.
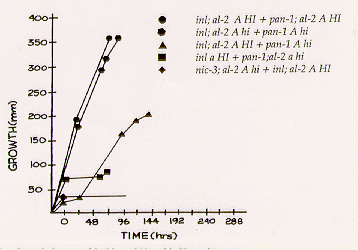
The effects of the alleles HI and hi in forced heterokaryons in
growth tubes are illustrated in Figure 1. Heterokaryons of the same HI or
hi genotype grow at nearly equal and fairly constant rates of approximately 4.0
mm/h at 30C. HI/hi heterokaryons exhibit various growth patterns. The most
common is growth rate like that of the HI/HI and hi/hi heterokaryons for
periods up to 36 h. Between 36 and 72 h the growth of these heterokaryons may slow, become
erratic, or cease.

Figure 1. Representative Growth Curves of Stable and Unstable Heterokaryons.
The conidia produced by duplicate sets of two unstable heterokaryotic cultures on minimal slants were plated to determine nuclear composition. The results are given in Table 1. It was evident that there were very few hi nuclei in the conidia produced by the HI/hi pairs.
Two unstable heterokaryons of pan-1 a; HI + inl; al-2 a; hi were set up in growth tubes with sampling ports to find out how soon the hi nuclei were lost. Conidia from sampling plugs as close as 10 mm from the inoculum site yielded only pan; HI colonies; no hi or heterokaryotic colonies were recovered.
Since selective inclusion of HI nuclei into conidia could explain the above results, conidia from another heterokaryon with the same components were plated, and single hyphal tips from the resulting colonies were excised and plated (Table 2).
These results suggest that the hi nuclei are greatly diminished in number soon after the heterokaryon is formed, and there is not a selective incorporation of HI nuclei into conidia.
Since the heterokaryons were produced with conidial suspensions, another possible explanation was that germinating HI conidia inhibited the germination of hi conidia. To check this possibility, we placed a series of two pairs of conidia in physical contact on slides coated with minimal agar, using a micromanipulator in a transfer hood. All three combinations were tested. The slides were incubated at 30C in moist chambers for about 6 h. After germination, the slides were photographed every 3 h.
Table 1. Indicated Nuclear Composition of Conidia Produced by Stable and Unstable Heterokaryons
| Heterokaryon |
Culture
Number |
Homokaryotic
Colonies
pan inl |
Heterokaryotic Colonies |
Total
Count |
Control
Count |
Error | |
| inl; HI 29a
+ |
1 | 37 | 70 | 277 | 384 | 358 | 7% |
| pan-1;HI 2a | 2 | 45 | 43 | 347 | 435 | 455 | 4% |
| inl;al-2;hi 3a
+ |
3 | 158 | 25 | 334 | 517 | 546 | 5% |
| pan-1;al-2;hi 21a | 4 | 33 | 22 | 330 | 385 | 443 | 13% |
| inl;HI 29a
+ |
5 | 171 | 471 | 6 | 648 | 642 | 1% |
| pan-1;al-2;hi 21a | 6 | 0 | 422 | 0 | 422 | 428 | 1% |
| pan-1;HI 2a
+ |
7 | 340 | 0 | 1 | 341 | 348 | 2% |
| inl;al-2;hi 3a | 8 | 604 | 5 | 4 | 613 | 641 | 4% |
Note: The nutritional markers of the heterokaryon components were the same, but the HI/hi components were switched in heterokaryons 5,6 and 7,8. Media were minimal, minimal plus necessary supplement for each nutritional mutant, and minimal plus both supplements for the control counts.
Table 2. Nuclear Composition of Hyphal Tips from HI/hi Heterokaryons
| Test Number | Medium | Number of Hyphal
Tips |
Number of Cultures
Produced |
| 1 | minimal | 127 | 0 |
| minimal +p +i | 102 | 80 | |
| 2 | minimal | 50 | 1 |
| minimal +p at 24 h | 50 | 22 | |
| 3 | minimal +i | 50 | 1 |
| minimal +p | 50 | 38 | |
| 4 | minimal +i | 50 | 0 |
| minimal +p | 50 | 47 |
Individual hyphal tips cut from 3-10 mm colonies of pan-1; HI+inl;al-2;hi on plates of minimal medium, and transferred to indicated media. In test 2, 100 hyphal tips were isolated. After 24 h at 30C, an appropriate amount of calcium pantothenate was added to 50. Abbreviations: p=pantothenate; i=inositol.
With HI/HI, we produced 4 heterokaryons in 24 attempts; with hi/hi, 5 in 17; and with HI/hi, 2 in 19. The stable heterokaryons covered a minimal plate in 48 h when transferred. The unstable heterokaryons stopped growing before the plate was covered. It appears that unstable heterokaryons are formed from conidia in much the same way as stable heterokaryons, and that HI conidia do not inhibit the germination of hi conidia.
In the above experiments, the agar film was thin enough to follow the germination
process. We were able to see that there was fusion or coalescence between the conidia
simultaneous with, or even before the germ tube(s) ever formed (Figure 2).
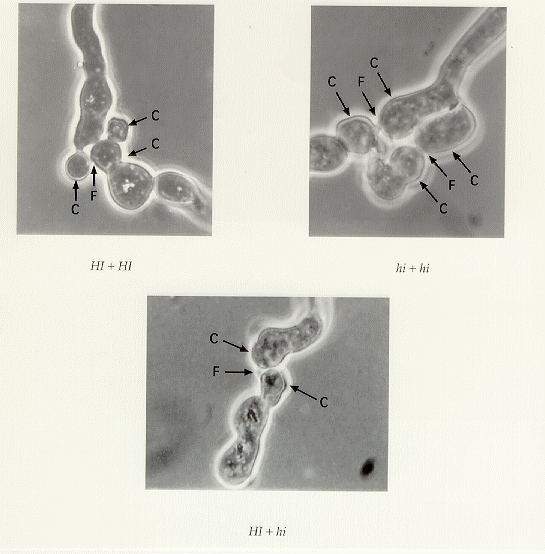
Figure 2. Heterokaryons formed by four conidia. Conidia photographed after nine hours' incubation. Arrows point to original conidia (C) and to fusions (F). Magnification 1300X.
No fusions between the germ tubes or young hyphae were observed. A search of the literature on conidial fusion was negative.
We checked for the presence of an agent in HI cytoplasm capable of inhibiting hi growth by microinjecting a crude HI extract into hi hyphal segments. [Such an extract would kill 87-100% of injected segments of a het cdE strain ( Wilson et al. 1963 Am. J. Bot. 48:299-305)]. The extract was non-toxic, and did not affect regeneration and growth. Experiments designed to detect a diffusible, inhibitory metabolite produced by HI cultures were negative, like those of Pittenger and Brawner.
Pittenger and Brawner controlled nuclear input ratios by relative numbers of viable conidia in the inoculum. We used a microsurgical approach to produce heterokaryons. A mixture of cytoplasm and nuclei from a donor was injected into a recipient hyphal segment, displacing approximately one-tenth of its contents. Three or four segments containing the injected one were cut out and transferred to a minimal plate or growth tube. This procedure introduced a maximum of about 10% donor nuclei in the injected cell, or 2.5% in a 3-cell section.
Heterokaryons were produced by microinjection in all four combinations of donor and
recipient genotypes; the fewest successes were with the hi HI
combination. Growth curves of some of the unstable heterokaryons are illustrated in Figure 3.
The initial growth rate of HI hi heterokaryons resembled that of unstable
heterokaryons formed by superimposed inocula. The growth rate of the two hi
HI heterokaryons was much lower, ranging from 0.21 to 0.83 mm/h, and they grew
less than 50 mm. The similarity of the growth curves within the two groups contrasts with those
of naturally formed unstable heterokaryons (Figure 1). In the formation of the artificial
heterokaryons there are two advantages -better control over initial nuclear ratios, and elimination
of hyphal fusions. The relative importance of each is not known.
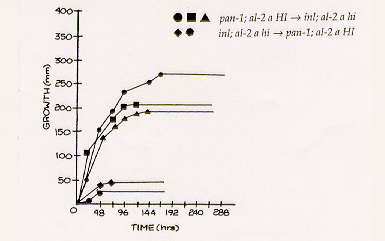
Figure 3. Growth Curves of Unstable Heterokaryons Produced by Nuclear Transplantation.
These experiments also illustrate the one difference found between the action of HI/hi and I/i genes. In these and all other het CDE strains tested, the ultimate outcome is unaffected by highly diverse initial nuclear ratios. In contrast, the i allele in het Cde is not suppressed if it is present in concentrations greater than 70%.
This apparent difference in the activity of HI/hi and I/i genes could result from differences in the two backgrounds, and the genes could be the same. We had HI/hi testers in het CDE, but OR-SL strains are het Cde. We would have to include a nutritional marker, and only one in eight of the progeny could be tested. However, we had some pure St. Lawrence wild types that were het CDe, (described on page 25) and with these, one-fourth of the progeny of a cross with het CDE could be tested.
We decided to make the crosses with known hi strains, since OR-SL strains were het i. An inl (37401)CDE hi was used as the parent in each cross. The results of these crosses are shown in Table 3. The results show that these OR-SL strains (and by extension, all OR-SL strains) are hi as well as i. There a number of possible explanations for these results, but the simplest is that HI/hi and I/i are the same or closely linked, since there was no sign of anything else segregating.
Table 3. Analysis of Crosses with St. Lawrence het CDe Wild Types
| HC 23-4 A CDe wt
X inl (37401)233 11-7 a CDE hi |
HC 23-8 a CDe wt
X inl (37401)233 ll-1 A CDE hi |
| 460 random ascospores | 440 random ascospores |
| 84% germination = 386 | 83% germination = 365 |
| inl 181 | inl 176 |
| inl CDe 94 | inl CDe 84 |
| inl CDE 87 | inl CDE 92 |
| inl CDE HI 0 | inl CDE HI 0 |
| inl CDE hi 87 | inl CDE hi 92 |
The HI gene has not been located. Eight ordered asci were analyzed and they all showed a 1:1 segregation of HI and hi. Five of the eight asci showed second division segregation, suggesting a centromere distance of at least 30 units. het i is on linkage group I or II (Part VII.D.2. FGSC Catalog).
The mechanism of HI/hi instability remains obscure. The experiments above seem to eliminate some explanations, leaving inhibition of hi nuclear replication and/or migration as possibilities.
With a few exceptions, the HI allele appears to be standard in RL strains, and hi is the predominant allele in OR-SL strains. The FGSC het testers are a mixture of the two alleles, since they were produced by crossing RL and OR-SL strains. Further, the original inl (37401) CD tester strains developed by Garnjobst we have found to be hi instead of HI (Garnjobst 1953 Am. J. Bot. 40: 607-614). With hindsight, her description of the behavior of heterokaryons in growth tubes is a description of HI/hi instability (Garnjobst 1955. Am. J. Bot. 42: 444-448). In developing the het C,D,E testers we used slant tests only.
A recent publication by Jacobson et al. (1995 Fungal Genet. Newsl. 42:34-40) describes erratic behavior of some FGSC het testers in growth tubes and crosses. They also point out that the het testers contain scot, which produces spreading colonial growth at 39C, but not at 25C or 30C. We have no explanation for the crosses, but we can speak to their heterokaryon problems. It looks as if they, like Dr. Garnjobst, have found the HI/hi instability gene.
We tested the strains Jacobson et al. used to form heterokaryons in growth tubes, using serial transfers with known het HI/hi strains. These tests showed that every incompatible combination but one involved a difference in het HI/hi genotype. The one exception was an OR mating type mutant, which Jacobson, et al. list as ad-3B cyh-1R am1 FGSC# 4564, and inl (37401)a. This pair was fully compatible in our tests, so the problem is possibly the mating type mutation. The multicent-4 A FGSC#6828 strain used by Jacobson et al. in crosses is not pure Oak Ridge, because it is HI instead of hi. Multicent-4 a FGSC#6829 is ambivalent, sometimes testing as HI; sometimes as hi in repeated tests. We have not encountered any ambivalent strains in het CDE.
Compatibility in stages II and III genes will not necessarily result in stable growth in a race tube. There are still potential stage III problems caused by inadequate hyphal fusions (Wilson and Dempsey, Fungal Genet. Newsl. 46), and the possibility of the OR nuclear ratio limitationaint existing in some FGSC het testers.
In RL strains, the instability genes have been used to produce temporary conidiation in aconidial strains, store the natural death mutant as a homokaryon, and simulate microinjections to produce heteroplasmy in a homokaryon.
Return to the FGN 46 Table of Contents