Detection of a protein phosphatase 2A holoenzyme in Neurospora crassa.
Einat Yatzkan1, Viktor Dombradi2 and Oded
Yarden1 - 1Department of Plant Pathology and Microbiology, Faculty
of Agricultural Food and Environmental Quality Sciences, The Hebrew University of Jerusalem,
Rehovot 76100, Israel; 2Department of Medical Chemistry, University Medical
School of Debrecen, Debrecen H-4026, Hungary.
Fungal Genetics Newsletter 46:32-33
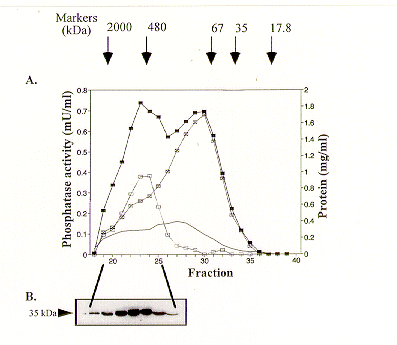
A high molecular mass (about 280 kDa) protein phosphatase 2A holoenzyme was detected in a crude N. crassa extract by gel filtration coupled with phosphatase activity assays. According to Western blot analysis the holoenzyme consists of a 36-kDa catalytic subunit complexed with an additional, yet unidentified, regulatory subunit(s).
In higher eukaryotes, the PP2A holoenzyme exists as a heterotrimer. The heterotrimer is comprised of a core complex consisting a 36-kDa catalytic subunit (PP2Ac) tightly associated with a 65-kDa regulatory subunit (Reg A). This dimeric core binds a third, variable, regulatory subunit (Reg B). The Reg B subunit has been shown to control enzyme activity and specificity (Mayer-Jaekel and Hemmings 1994 Trends Cell Biol. 4: 287-291). Previously, we have cloned pph-1, the gene encoding the PP2Ac, and demonstrated its essential role in N. crassa (Yatzkan and Yarden 1995 Curr. Genet. 28: 458-466). We have shown that lower levels of pph-1 transcript and lower PP2A activity conferred increased sensitivity to phosphatase inhibitors and reduction in hyphal growth (Yatzkan et al., 1998 Mol. Gen. Genet. in press). Though the N. crassa PP2Ac has been purified and biochemically characterized (Szoor et al., 1995 Comp. Biochem. Physiol. 112B: 515-522), the quaternary structure of PP2A has not been studied in a filamentous fungus. In the present communication we describe the fractionation and partial purification of the N. crassa PP2A holoenzyme as well as the immuno-detection of the PP2Ac in the fractionated enzyme complex.
Protein extraction from N. crassa wild-type strain 74-OR23-1A (FGSC987) and phosphatase assays were performed as described earlier (Szoor et al., 1995 Comp. Biochem. Physiol. 112B: 515-522), with a minor modification. Namely, the extraction buffer was amended with 0.3 M sucrose in order to stabilize the PP2A holoenzyme. The 15,000g supernatant of the mycelial extract (1 ml) , was loaded on a Superdex 200HR 10/30 (Pharmacia) column. The sample was fractionated on a Pharmacia FPLC system using an imidazole buffer (50 mM, pH 7.4) containing 5 mM EDTA, 10 mM -mercapthoethanol and 100 mM NaCl, in the cold at a flow rate of 500 µl/min. Void volume was determined with Blue Dextran 2000 (Pharmacia). Fractions were assayed for protein phosphatase activity in the absence or in the presence of specific phosphatase inhibitors. Fractions 19-25 (0.5 ml each) were precipitated with 1 ml acetone and the precipitate was subsequently resolved in a 10% SDS polyacrylamide gel. Anti-PP2Ac antibodies raised against a synthetic peptide corresponding to residues 296-309 of the C-terminal region of human PP2A (Upstate Biotechnology) were diluted 5,000-fold and were used in Western blot analysis as described by Szoor et al., (1995 Comp. Biochem. Physiol. 112B: 515-522).
The size of the N. crassa PP2A holoenzyme was calculated to be approximately 280 kDa based on the elution profile of the okadaic acid-sensitive phosphatase active fractions (Figure 1A). The highest PP2A activity was measured in fractions 21-25 with peak activity in fractions 23-24. These results correlate well with the immuno-detection of PP2Ac in the same fractions (Figure 1B). Thus practically all of the PP2A activity and the PP2Ac protein is present in a high molecular mass holoenzyme. Since the molecular mass of PP2Ac is 36 kDa, the holoenzyme must exist as a complex between the catalytic subunit and other, yet to be identified regulatory submit(s). The phosphatase activity sensitive to the inhibitor-2 protein can be attributed to protein phosphatase 1 (PP1). Even though it is evident that a portion of PP1 is eluted in the high molecular mass range, most of the PP1 activity appears in the lower molecular mass fractions (Figure 1A), as expected under these purification conditions, which do not favor the maintenance of a stable PP1 complex.
Further characterization of the putative Reg A and Reg B subunits of the N. crassa PP2A holoenzyme along with dissection of the actual components of the functional complex(es) described here will serve to complement the genetic data which is accumulating concerning this protein phosphatase.
Acknowledgments
We thank Mrs. Margit Biro for her technical assistance. This research was supported by
BARD, The United States-Israel Binational Research and Development Fund, by the Hungarian
Research Foundation (OTKA 22675), and by an Israeli-Hungarian Bilateral
Scientist Exchange grant ISR-2/96.

Return to the FGN 46 Table of Contents