Expression and Visualization of Red Fluorescent Protein (RFP) in Neurospora crassa
Michael Freitag# and Eric U. Selker
Institute of Molecular Biology, University of Oregon, Eugene, OR 97403-1229
# corresponding author.
Fungal Genetics Newsletter 52:14-17
We report the expression of Discosoma red fluorescent protein (RFP) and RFP fusion proteins in Neurospora crassa. RFP was expressed under the control of the Neurospora ccg-1 promoter in transformants with single copies integrated at the his-3 locus by gene targeting. Because this RFP gene, tdimer2(12), contains a 677 bp direct tandem repeat of dsRed, RFP constructs underwent RIP at high frequency in rid+ strains. Fusion proteins of RFP to the amino terminus of Neurospora heterochromatin protein 1 (HP1) were localized to heterochromatic foci in Neurospora nuclei, consistent with prior findings with carboxy-terminal HP1-GFP fusion proteins.
Heterologous expression of jellyfish Green Fluorescent Protein (GFP) has become an indispensable tool for biological studies. We and others have reported successful expression of GFP in Neurospora (Freitag et al., 2001; 2004a; 2004b; Fuchs et al., 2002). Many applications of live cell imaging require simultaneous observation of more than one protein, however, prompting this study. Some pairs of fluorescent fusion proteins (e.g., green and red, or cyan and yellow) are typically observed simultaneously, because their excitation and emission spectra are well separated, but multi-color imaging is feasible with appropriate hardware and software. Here we describe the development of Neurospora plasmids for the expression of fusion proteins with an RFP gene that was derived from dsRed (or drFP583), first cloned from a Discosoma coral species (Matz et al., 1999).
We began by testing transcriptional fusions of Neurospora promoters to an enhanced version of dsRed, tdimer2(12) (Campbell et al., 2002). We chose this version because it matures more quickly and is brighter than dsRed, properties achieved by engineering and fusing two dsRed genes and separating them by a 42 bp spacer (which encodes the peptide linker “GHGTGSTGSGSSGT”), thus generating an almost perfect 677 bp direct repeat (Campbell et al., 2002). We expected that Neurospora transformants with this gene would undergo RIP at high frequencies when crossed in wild type (i.e., rid+) strains.
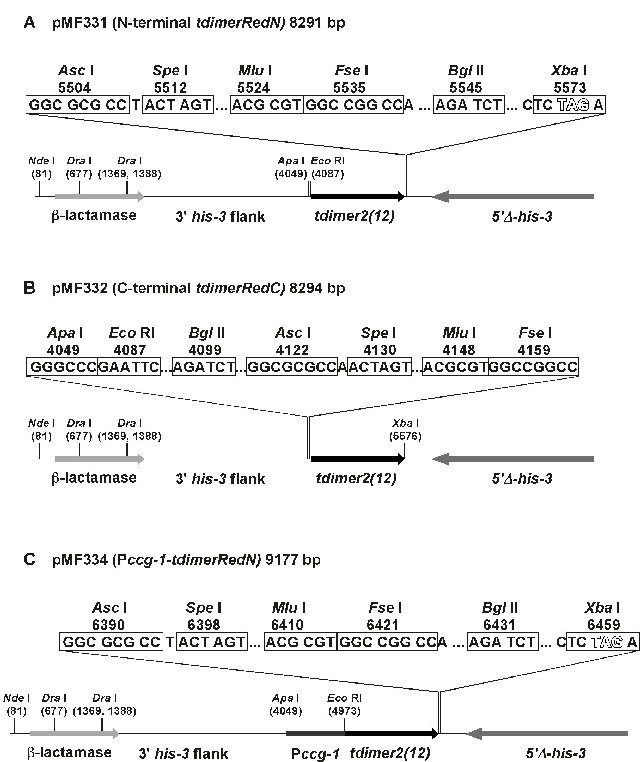
The tdimer2(12) gene was exised from pdNT and pdCT (generously provided by Melissa Rolls, Univ. of Oregon) by digestion with Apa I and Xba I and inserted into pBM61 (Margolin et al., 1997) to yield pMF331 and pMF332, respectively (Fig. 1). In pMF331, a plasmid to generate amino-terminal RFP fusions, a consensus Kozak translation initiation sequence precedes the 1391 bp tdimer2(12) gene, which is followed by a 12 bp glycine linker (“GSGG”) and a 63 bp region with multiple rare cloning sites (Fig. 1A). The ApaI and EcoRI sites can accept promoters for the genes to be tested. In pMF332, a plasmid to express carboxy-terminal RFP fusions, the multiple cloning site is followed by the glycine linker and the tdimer2(12) gene (Fig. 1B). To test amino-terminal RFP fusions in the pMF331 plasmid, the Neurospora ccg-1 promoter from pMF272 (Freitag et al., 2004a) was amplified by PCR with Pfu polymerase and inserted between the ApaI and EcoRI sites of pMF331 to yield pMF334 (Fig. 1C). Thethree plasmids are available from the Fungal Genetic Stock Center and their complete sequences have been submitted to the GenBank database (accession numbers: pMF331, DQ250997; pMF332, DQ250998; pMF334, DQ250999).
We transformed a N. crassa his-3 strain (N623; FGSC# 6103) and rid his-3 strain (N2240; FGSC# 9014) with pMF334 by electroporation (Margolin et al., 1997). Transformants were selected on Vogel’s minimal medium with sorbose to induce colonial growth. Under these conditions, expression of RFP was detected with an Olympus SZX12 fluorescence stereo microscope in macroconidia and hyphae after three to five days in heterokaryotic prototrophic transformants. Fluorescent strains were picked to slants with minimal sucrose medium; fluorescence in aerial hyphae was detectable after overnight incubation at 32 C.
For conventional epifluorescence microscopy, Neurospora conidia were spotted on microscope slides in droplets containing minmal medium with 1.5 % sucrose; hyphae were examined on agar blocks (Freitag et al., 2004b). We used a Zeiss Axioplan 2 microscope equipped with UV and FITC filtersets and images were captured with an Orca II digital camera (Hamamatsu, San Jose, CA) and Openlab software (Improvision, Coventry, UK). Images were processed with Photoshop software (version 4.0; Adobe, San Jose, CA). RFP accumulated in macroconidia, allowing visualization in germ tubes and young hyphae after 3 - 12 hours (Fig. 2).
We expected to observe high RIP frequencies in crosses of primary transformants because of the tandem duplication in tdimer2(12). Indeed, 99 of 100 His+ progeny of one N623-derived transformant crossed to a his-3 (N625; FGSC# 6525) strain were RFP-. Crosses of N2240-derived rid RFP+ transformants to a rid his-3 strain (N2257; FGSC# 9015) yielded only RFP+ His+ progeny, as expected because RIP is abolished in homozygous rid crosses (Freitag et al., 2002). Subsequent crosses of homokaryotic rid his- 3+::tdimer2(12) RFP+ strains with rid+ strains yielded no RFP+ progeny (0/100). This confirmed our previous finding that rid shows little or no effect on RIP frequency in heterozygous crosses (Freitag et al., 2002). We note that this RFP construct could serve as a sensitive locus to screen for RIP-deficient mutants. When RFP+ progeny are desired, however, we suggest that investigators introduce RFP constructs in rid strains to avoid RIP.
To test simultaneous live cell imaging of GFP- and RFP-tagged versions of the same protein, we generated a translational fusion of RFP with Neurospora heterochromatin protein 1 (HP1). We previously showed that carboxy-terminal GFP fusions of the HP1 gene (hpo) localized to heterochromatin and complemented DNA methylation defects and growth phenotypes of hpo null mutants (Freitag et al., 2004b). We wanted to verify that neither the position nor the type of tag interfere with HP1 localization.
We inserted an AscI - BamHI segment of the hpo coding region between the AscI and BglII sites of pMF334 to generate a tdimer2(120-hpo fusion (pMF344). We targeted this construct to his-3 by electroporation of N623 and N2240 strains. RFP-HP1 was targeted to heterochromatin (Fig. 3, RFP), as expected from previous results with the HP1-GFP fusion protein (Freitag et al., 2004b). To observe co-localization of RFP- and GFP-tagged HP1, we generated heterokaryons by growing RFP-HP1 transformants with an HP1-GFP strain (N2540; Freitag et al., 2004b). Unforced heterokaryons were usually established overnight. Conidia with both RFP and GFP fluorescence were re-grown and observed after overnight growth at 32 C on Petri plates with sucrose as the carbon source. GFP- and RFP-tagged HP1 fusion proteins were localized to heterochromatic regions and their distribution was largely congruent (Fig. 3). These results indicate that neither the location of the tag (i.e., amino- vs. carboxy-terminal) nor the type of tag (coral RFP vs. jellyfish GFP) altered the expected localization of Neurospora HP1.
Figure 2. Expression of cytosolic RFP in N. crassa. Neurospora hyphae (left) and conidia (right) were examined by epifluorescence microscopy (see text). Even heterokaryotic transformants generated by insertion of pMF334 into the his-3 locus gave strong fluorescence (exposure times at 100% lamp power and 70% gain are shown). We noticed that under these conditions RFP bleached faster than GFP constructs (Freitag et al., 2004a; 2004b).
Figure 3. Colocalization of amino-terminal RFP-HP1 and carboxy-terminal HP1-GFP fusion proteins in heterochromatic regions. Conidia were examined by epifluorescence microscopy (see text). Heterochromatic foci in nuclei were detected in heterokaryotic strains expressing RFP-HP1 (RFP) and HP1-GFP (GFP), and overlapped with foci detected by staining with the DNA dye Hoechst 33258 (data not shown). The outline of nuclei is visible in the RFP and GFP panels of the lower right conidium with four nuclei. RFP-HP1 and HP1-GFP exhibit largely congruent distribution (see white arrows in the RFP, GFP and merged panels. Because of rapid nuclear movement, foci do not always align perfectly (red and green arrows in the RFP, GFP and merged panels). Panels are composites of three images.
In conclusion, expression of RFP in Neurospora can be achieved by using a strong fungal promoter. The cytosolic RFP expression profile was as expected from previous studies with the ccg-1 promoter (Freitag et al., 2004a). We generated a set of RFP plasmids that allow the expression of amino- or carboxy-terminal fusion proteins under the control of the ccg-1 or other (e.g., endogenous) promoters. All three plasmids are designed for targeting to the Neurospora his-3 locus but can also be used in co-transformation experiments with other selectable markers (M. Freitag and E.U. Selker, unpublished results). In combination with our GFP constructs (Freitag et al., 2004a; 2004b), these RFP constructs should prove useful for studies in which two different fluorescent tags are required.
Acknowledgements:
We thank Melissa Rolls for providing plasmids pdNT and pdCT, which contain an amino- and carboxy-terminal tdimer2(12) fusion, respectively. Funding for this research was provided by US Public Health Service grant GM35690 from the National Institutes of Health and National Science Foundation grant MCB-0131383 to E.U.S.
References:
Campbell, R.E., Tour, O., Palmer, A.E., Steinbach, P.A., Baird, G.S., Zacharias, D.A., Tsien, R.Y., 2002. A monomeric red fluorescent protein. Proc. Nat. Acad. Sci. (USA) 99: 7877-7882.
Freitag, M., Ciuffetti, L.M., Selker, E.U., 2001. Expression and visualization of Green Fluorescent Protein (GFP) in Neurospora crassa. Fungal Genet. Newslett. 48: 15-19.
Freitag, M., Williams, R.L., Kothe, G.O., Selker, E.U., 2002. A cytosine DNA methyltransferase homologue is essential for repeat-induced point mutation in Neurospora crassa. Proc. Nat. Acad. Sci. (USA) 99: 8802-8807.
Freitag, M., Hickey, P.C., Raju, N.B., Selker, E.U., Read, N.D., 2004a. GFP as a tool to analyze the organization, dynamics and function of nuclei and microtubules in Neurospora crassa. Fung. Genet. Biol. 41: 897-910.
Freitag, M., Hickey, P.C., Khlafallah, T.K., Read, N.D., Selker, E.U., 2004b. HP1 is essential for DNA methylation in Neurospora. Mol. Cell 13: 427-434.
Fuchs, F., Prokisch, H., Neupert, W., Westermann, B., 2002. Interactions of mitochondria with microtubules in the filamentous fungus Neurospora crassa. J. Cell Sci. 115: 1931-1937.
Margolin, B.S., Freitag, M., Selker, E.U., 1997. Improved plasmids for gene targeting at the his-3 locus of Neurospora crassa by electroporation. Fungal Genet. Newslett. 44: 34-36 (and correction in FGN 47: 112).
Matz, M.V., Fradkov, A.F., Labas, Y.A., Savitsky, A.P., Zaraisky, A.G., Markelov, M.L., Lukyanov, S.A., 1999. Fluorescent proteins from nonbioluminescent Antozoa species. Nature Biotech. 17: 969-973.