Influence of Lithium ions on conidiophore size in Neurospora crassa
Bodil Aase, Ingunn W. Jolma and Peter Ruoff#
Department of Mathematics and Natural Science, University of Stavanger, N-4036 Stavanger, Norway
#corresponding author. email: peter.ruoff@uis.no.
Fungal Genetics Newsletter 53:34-36
Lithium (Li) ions are known to affect Neurospora crassa’s growth speed and circadian clock period, while elevated temperatures abolish these influences. We wondered whether Li has also an effect on conidia size. We used cryo-SEM to investigate this question and report here the results of 1720 measurements showing that at 20°C the long and short conidial axes are significantly reduced at high Li concentrations (10-15 mM), while the ratio between the long and short axes remains approximately constant. An increased temperature (30°C) appears to abolish the Li effect on conidia size.
Lithium (Li) has a profound influence on Neurospora crassa’s growth rate and circadian period (Engelmann 1987; Davis 2000; Dunlap and Loros 2004). Typically, at extracellular concentrations of 10 mM LiCl, the growth rate is significantly reduced and the circadian clock begins to get disrupted (Engelmann 1987; Lakin-Thomas 1993; Jolma et al. 2006). Interestingly, increased temperature can abolish the Li effect, possibly by an increased dissociation between Li and its assumed targets (Jolma et al. 2006). Because of the macroscopically distinct differences in conidiation when Neurospora is grown in the presence or absence of Li, we wondered whether there might be also a difference in microscopic conidiation, for example conidia size. In order to answer this question, we performed a study using a Zeiss Supra VP35 scanning electron microscope (SEM) with a Polaron cryo stage.
The bd a strain (FGSC #1859) was grown on Petri dishes in LD (12h:12h) at 20°C or 30°C using Vogel’s medium as previously described (Jolma et al. 2006). Li was added to the medium as LiCl. Samples were taken from Petri dishes that showed approximately the same amount of total growth (=growth speed X growth time). Because of the dependence of the speed of this organism’s growth on temperature and LiCl concentrations (Jolma et al. 2006) the growth time prior sampling varied as shown in Table 1.
Table 1: Growth times prior analysis
T, °C |
LiCl, mM |
GT, days |
20 |
0 |
3 |
20 |
5 |
3 |
20 |
10 |
10 |
20 |
15 |
10 |
30 |
10 |
1 |
T: temperature; LiCl: concentration of LiCl;
GT: growing time
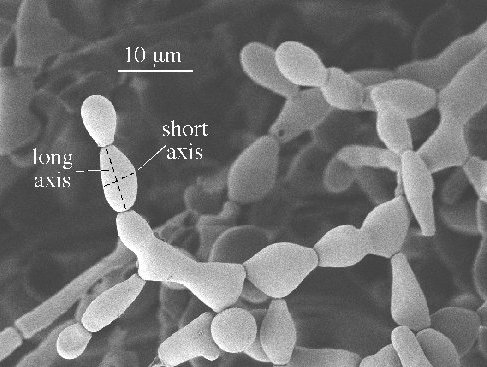
Figure 1. Measurement of conidia size as long and short axis, l and s, respectively.
The picture shows conidia grown at 20°C with 15 mM Li.
Samples were cut out using a cork-borer covering the growth zone and approximately 5 mm behind the growth zone. The cut-out samples were put on a Al-stub that had glue with colloidal graphite on its surface. The glued sample was rapidly frozen in nitrogen slush and then transferred to the pre-cooled SEM stage. Samples were then coated with a gold-palladium alloy for 80 seconds. In the SEM analysis the ‘In-Lens’ detector of the microscope was used. Conidia have an ellipsoidal-like shape and the long and short axes l and s (Fig. 1), which could be determined directly on the sample, were used as an approximate measure for conidia size. Overall, a total of 1720 measurements were performed with different LiCl concentrations. The results show (Table 2) that at 20°C high LiCl concentrations (up to 15 mM) reduce the long and short conidial axes by up to circa one third. However, the ratio between the long and short axes remains approximately constant and was found to lie between 1.5-1.7 (Table 2). A t-test showed that the differences in short and long axes observed for 0 mM, 5 mM and 10/15 mM Li at
Table 2: Long and short axes as a function of LiCl concentration and temperature
T, °C |
LiCl, mM |
N |
s, mm |
SD (s), mm |
l, mm |
SD (l), mm |
ratio l/s |
20 |
0 |
307 |
6.0 |
1.4 |
8.7 |
1.9 |
1.5 |
20 |
5 |
278 |
4.5 |
1.0 |
7.3 |
1.4 |
1.6 |
20 |
10 |
304 |
3.8 |
0.8 |
6.6 |
1.4 |
1.7 |
20 |
15 |
336 |
3.8 |
0.8 |
6.5 |
1.3 |
1.7 |
30 |
10 |
495 |
6.0 |
1.3 |
8.7 |
2.1 |
1.5 |
T: temperature; LiCl: concentration of LiCl ; N: number of measurements; l: long axis;
s: short axis; SD: standard deviation; ratio l/s: ratio between long and short axes.
20°C are statistically significant (P < 0.0001). No significant differences in l or s values were observed when using 10 or 15 mM Li. At 10-15 mM Li we also observed the occurrence of more non-mature conidia, i.e., conidia that were still connected by minor constrictions (Springer and Yanofsky 1989). This observation was difficult to assess quantitatively and we did not further investigate how fast maturation of conidia occurred in the presence of LiCl. However, we cannot exclude the possibility that the smaller conidia sizes observed in the presence of LiCl are due to a slower speed in maturation compared to non-Li conditions.
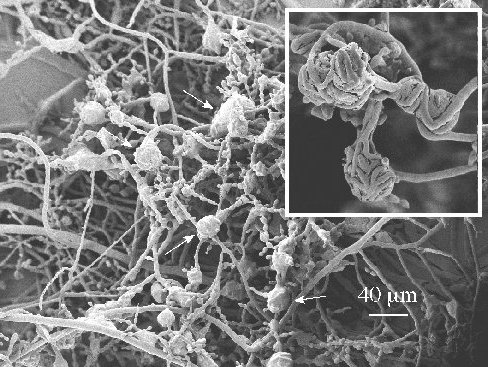
Figure 2. At 30°C, independent of Li con-centrations, the appearance of proto-perithecia-like structures was observed (indicated by arrows). The inset shows a close-up picture of these structures.
It was recently found that an increase in temperature can abolish the Li-effect on Neurospora’s growth rate and circadian period length (Jolma et al. 2006). A similar effect was now observed with
conidia size, i.e., at 30°C LiCl did not lead to a decrease in l or s as found for 20°C (Table 2). Somewhat to our surprise, however, 30°C triggered the appearance of what seemed to be protoperithecia or “false perithecia”, which were not observed at 20°C (Fig. 2, see also David D. Perkins: “Hints and precautions for the care, feeding and breeding of Neurospora,” http://www.fgsc.net/methods/perkins.html). Further experiments showed that the formation of these structures at 30°C were Li-independent. We hope to characterize these structures in a later study.
Acknowledgements:
We thank Ingunn Cecilie Oddsen and Ola Risvik for help using the Zeiss Supra 35VP microscope.
References:
Davis, R.H. 2000. Neurospora. Contributions of a Model Organism. Oxford University Press, New York.
Dunlap, J.C., and J.J. Loros. 2004. The Neurospora circadian system. J. Biol. Rhythms. 19: 414-424.
Engelmann, W. 1987. Effects of Lithium Salts on Circadian Rhythms. In: Halaris A. (ed) Chronobiology and Psychiatric Disorders. Amsterdam, Elsevier pp. 263-289.
Jolma, I.W., G. Falkeid, M. Bamerni, and P. Ruoff. 2006. Lithium leads to an increased FRQ protein stability and to a partial loss of temperature compensation in the Neurospora circadian clock. J. Biol. Rhythms. 21: 327-334.
Lakin-Thomas, P.L. 1993. Evidence against a direct role for inositol phosphate metabolism in the circadian oscillator and the blue-light signal transduction pathway in Neurospora crassa. Biochem. J. 292 ( Pt 3): 813-818.
Springer, M.L., and C. Yanofsky. 1989. A morphological and genetic analysis of conidiophore development in Neurospora crassa. Genes Dev. 3: 559-571.
Return to the FGN 53 Table of Contents