Non-circadian light inducible rhythm in Aspergillus nidulans
Sijmen E. Schoustra*, Marijke Slakhorst, Rolf F. Hoekstra and Alfons J.M.
Debets
Laboratory of Genetics, Wageningen University, Arboretumlaan 4, 6703 BD
Wageningen, the Netherlands.
* Present address: Biology Department, University of Ottawa, Canada, ON K1N 6N5.
Fungal Genetics Newsletter 53:23-25
Aspergillus nidulans grown under standard laboratory conditions does not show circadian rhythmic growth. The presence of a circadian clock was demonstrated in A. nidulans at the level of gene expression (Greene et al. 2003), but a visually observable rhythm is lacking. We recently observed a remarkable rhythmic growth pattern in the formation of conidiospores and ascospores in a fludioxonil resistant mutant of A. nidulans (fldA1) grown in a dark incubator. This is reminiscent of a circadian rhythm. We found however that our observed rhythm is induced by light (leaking into the ‘dark’ incubator) and is not self-sustainable. In absolute darkness or constant light the rhythm is lost; therefore, we conclude that the rhythm is not a true intrinsic circadian rhythm.
Circadian rhythms in biological activities are wide spread in nature to keep organisms in synchrony with the 24-hour day-night cycle on earth ENRfu(Bell-Pedersen et al. 2005). In fungi, circadian rhythms are studied mainly with the use of the model organism Neurospora crassa. In N. crassa, the phenotypic manifestation as well as the genetic background of circadian rhythms has been extensively studied ENRfu (Loros and Dunlap 2001). The circadian rhythm can be observed in asexual spore production. The band (bd) mutation enhances the visualization of the rhythm in N. crassa, as it renders the cells less sensitive to high CO2 levels in race tubes and Petri dishes; high CO2 levels inhibit the formation of conidia ENRfu (Bell-Pedersen 2000; Sargent et al. 1966).
In 2003, the presence of a circadian clock was demonstrated in Aspergillus flavus and Aspergillus nidulans ENRfu(Greene et al. 2003). In A. flavus, an endogenous circadian rhythm in sclerotium production was observed. However, in A. nidulans only a circadian rhythm in expression of genes homologous to circadian rhythm regulation genes in A. flavus and N. crassa was observed and an easily observable rhythm in phenotype is lacking.
We here present the results of a (physiological) characterization of rhythmic growth exhibited by a fludioxonil resistant mutant (fldA1) of a velvet (veA1) strain of Aspergillus nidulans. This mutant was identified while investigating the development of resistance to the fungicide fludioxonil and subsequent compensatory evolution in A. nidulans ENRfu(Schoustra et al. 2006; Schoustra et al. 2005). We observed that this mutant strain formed a banding pattern when grown in a incubator at 37 0C with a clear day/night rhythm in the formation of conidiospores and ascospores respectively (Figure 1). This was unexpected since velvet strains of A. nidulans are known not to exhibit any light inducible or circadian rhythm in the formation of conidiospores and ascospores ENRfu(Kafer 1977; Timberlake 1990).
We therefore investigated whether the rhythm found in our A. nidulans mutant strain (veA1, fldA1) is circadian ENRfu(Johnson et al. 2003; Loros and Dunlap 2001). We tested for the following characteristics: (1) Is the rhythm self-sustainable and persistent in the absence of temporal signals such as light/dark or temperature oscillations? (2) Can the rhythm be entrained, or reset, by external signals?
Strains. The Aspergillus nidulans strains used in this study were derived from the Glasgow collection ENRfu (Clutterbuck 1974); see Table 1. For the experiments, we used a fldA1 mutant strain exhibiting a light inducible rhythm (WG622) and a control strain not exhibiting the rhythm (WG562). This mutant strain was derived from a fludioxonil resistant mutant (WG561), which underwent compensatory evolution for 3000 generations ameliorating costs associated with fungicide resistance ENRfu (Schoustra et al. 2006). The non-compensated resistant mutant (WG561) exhibits the rhythm; however the compensated mutant does so in a more pronounced way as is shown in Figure 1.
Growth media and conditions. Minimal medium (MM) was used ENRfu(Clutterbuck 1974), supplemented with lysine (to a final concentration of 2 mmol/l). The sugar concentration (100%, 30% and 10% of 15 g/l) was varied to assess its influence on the banding pattern. Strains were grown at 37 oC. To facilitate experiments over a ten-day period, race tubes and large size Petri dishes were used.
Table 1. Strains used in this study and their genotypes.
|
Strain |
Description |
Genotype |
|
WG044 |
Velvet+ strain used to construct a fldA1; veA1+ strain |
veA1+; ornB7; fwA1 |
|
WG562 |
Fungicide sensitive strain |
lysB5; veA1 |
|
WG561 |
Mutant strain resistant to fludioxonil |
fldA1; lysB5; veA1 |
|
WG615 |
Fungicide resistant strain, used to construct diploids |
wA3; fldA1; pyroA4; veA1 |
|
WG622 |
Mutant strain resistant to fludioxonil with compensation of fitness costs |
fldA1; lysB5; flc8; veA1 |
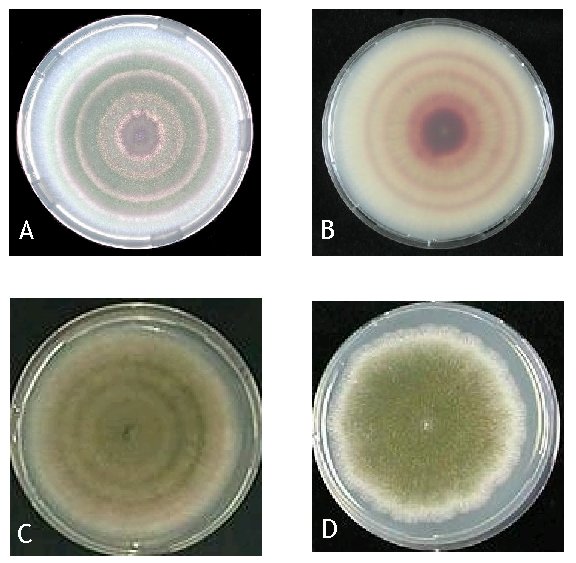
Greene et al (2003) have shown that a number of genes homologous to genes associated with circadian rhythms in other organisms show an autonomous cycle in expression in Aspergillus. In A. nidulans, no obvious rhythms in development are apparent. The fludioxonil resistance mutation (fldA1) in A. nidulans that we recently identified ENRfu(Schoustra et al. 2006; Schoustra et al. 2005), however, gives rise to a clear rhythmic growth pattern when a fldA1 strain is grown in a closed incubator (in ‘darkness’) at 37 0C. The rhythm (Figure 1) shows a clear day/night fluctuation in asexual and sexual spore formation, which is atypical for A. nidulans and is not seen in the ancestral (fldA1+) strain. Very clearly, the fungus produces the asexual conidiospores during "daytime" and switches to the production of sexual spores during the "night". During day the chances of dispersal of the airborne asexual spores are highest due to more air movement, this could explain this switch between sexual and asexual sporulation.
To verify whether this observation is due to a circadian rhythm or rather just due to external stimuli we did the following experiments. First, we checked whether the rhythm occurs independently of external temporal signals. Here, we found that the rhythmic growth pattern is not observed for cultures grown in absolute darkness (packed in aluminum foil) or constant light (full light always on in the incubator). The amount of light needed for induction of the banding pattern is remarkably low however: the light passing through a closed door of an incubator is sufficient to give the observed rhythm with a periodicity of about 24 hours.

Figure 1. Aspergillus nidulans strains. Panels (a) and (b) WG622 (see Table 1) demonstrating an alternation in the formation of conidiospores and ascospores induced by a Light/Dark cycle (12L/12D). Panel (a) shows the surface of the colony growing on solid medium in a Petri dish; panel (b) shows the same colony photographed through the bottom of the Petri dish. There are clear sections of the colony were asexual sporulation predominates (green conidia) in the light period of the cycle. The parts with brown/red color contain the sexual fruiting bodies (cleistothecia with red ascospores) that are formed in the dark period of the cycle. Panel (c) shows a diploid strain heterozygous for the fldA1 mutation (WG622//615), showing the banding pattern in a less clear way. Panel (d) shows an fldA1+ strain (WG562) grown under the same conditions as WG622 and WG622//615.
Next, we tested for induction of the banding pattern by growing the mutant strain (WG622) under various Light/Dark (L/D) cycles of 10 hours light and 10 hours dark (10L/10D), 12L/12D and 18L/18D. This resulted in the formation of the banding pattern consisting of clear rings of cleistothecia and conidiospores in every L/D cycle (Figure 1). When growing the mutant strain in total darkness for three days, a switch to a L/D cycle induces the formation of the banding pattern from then on. A switch from an L/D cycle to continuous light or continuous darkness ends the formation of any banding pattern.
When a L/D cycle is applied, the strain exhibits the banding pattern at all temperatures tested (27 oC, 30 oC, 33 oC, 37 oC, 39 oC and 40.5 oC) and at all sugar concentrations (MM with 1%, 0.3% and 0.1% glucose). Diploids heterozygous for the fludioxonil resistance mutation (fldA1), exhibit the banding pattern in a less pronounced way (Figure 1c); homozygous diploids do show the banding pattern in a very clear way. In A. nidulans light dependence of conidiation is abolished by a mutation in the velvet gene (our mutant strain also carries the veA1 mutation), which allows conidiation to occur in the absence of light ENRfu(Mooney and Yager 1990). We tested fldA1 in a velvet+ background (by crossing our mutant strain WG622 with velvet+ strain WG044) and observed that a veA1+, fldA1 strain also exhibits the banding pattern.
A defining property of a circadian rhythm is that it is sustained in the absence of any environmental cycle (a light/dark in this case). We found that the rhythm expressed by our mutant strain is not self-sustainable: it is lost when cultures are placed in constant light or constant darkness, even after six cycles of LD. Therefore, the rhythm in sexual and asexual development seen in this mutant may be a direct response to light/darkness not reflecting a true intrinsic circadian rhythm.
Acknowledgements
We wish to thank Deborah Bell-Pedersen for stimulating comments and suggestions. This research was funded by the Netherlands Organization for Scientific Research (NWO).
References
Bell-Pedersen, D. 2000. Understanding circadian rhytmicity in Neurospora crassa: From behavior to genes and back again. Fungal Genetics and Biology 29:1-18.
Bell-Pedersen, D., V. M. Cassone, D. J. Earnest, S. S. Golden, P. E. Hardin, T. L. Thomas, and M. J. Zoran. 2005. Circadian
rhythms from multiple oscillators: lessons form diverse organisms. Nature Reviews Genetics 6:544-556.
Clutterbuck, A. J. 1974. Aspergillus nidulans. Pp. 447-510 in P. C. King, ed. Handbook of Genetics. Plenum Press, New York.
Greene, A. V., N. Keller, H. Haas, and D. Bell-Pedersen. 2003. A circadian oscillator in Aspergillus spp. regulates daily development and gene expression. Eukaryotic Cell 2:231-237.
Johnson, C. H., J. A. Elliott, and R. Foster. 2003. Entrainment of circadian programs. Chronobiology International 20:741-774.
Kafer, E. 1977. Meiotic and mitotic recombination in Aspergillus and its chromosomal aberrations. Advances in Genetics 19:33-131.
Loros, J. J., and J. C. Dunlap. 2001. Genetic and molecular analysis of circadian rhythms in Neurospora. Annual Reviews in Physiology 63:757-794.
Mooney, J. L., and L. N. Yager. 1990. Light is required for conidiation in Aspergillus nidulans. Genes and Development 4:1473-1482.
Sargent, M. L., W. R. Briggs, and D. O. Woodward. 1966. Circadian nature of a rhythm expressed by an invertaseless strain of Neurospora crassa. Plant Physiology 41:1343-1349.
Schoustra, S. E., A. J. M. Debets, S. M. Slakhorst, and R. F. Hoekstra. 2006. Reducing the cost of resistance; experimental evolution in the filamentous fungus Aspergillus nidulans. Journal of Evolutionary Biology In press
Schoustra, S. E., S. M. Slakhorst, A. J. M. Debets, and R. F. Hoekstra. 2005. Comparing artificial and natural selection in rate of adaptation to genetic stress in Aspergillus nidulans. Journal of Evolutionary Biology 18:771-778.
Timberlake, W. E. 1990. Molecular genetics of Aspergillus development. Annual Reviews in Genetics 24:5-36.
Return to the FGN 53 Table of Contents