Complementation of un-16 and the development of a selectable marker for transformation of Neurospora crassa
Kevin McCluskey, Sheera A. Walker, Rachel L. Yedlin, David Madole and Michael Plamann
School of Biological Sciences, University of Missouri-Kansas City, Kansas City, Missouri 64110
Fungal Genetics Newsletter 54: 9-11
Although nearly sixty temperature-sensitive lesions have been mapped in Neurospora crassa, most of their functions have not been identified. These loci are called unknown (un). As part of an effort to identify the open reading frame associated with one of these, we undertook to walk to un-16 using the complementation of temperature-sensitivity as a selection. Cosmids complementing un-16 were identified and the un-16 gene was subcloned. DNA sequence analysis of un-16 revealed that it encodes the highly conserved S9 protein of the 40S ribosomal subunit. This gene has proven useful as a selectable marker and may provide a simple mechanism for the controlled alteration of protein synthesis in N. crassa.
Temperature-sensitive mutants can be very useful tools for various studies, because they allow temperature-controlled inactivation of essential cellular processes such as DNA, RNA and protein syntheses. An extensive set of temperature-sensitive N. crassa mutants is available from the Fungal Genetics Stock Center (FGSC); however, only a few of these mutants have been studied in depth (Perkins et. al., 2001). To enhance the usefulness of this potentially valuable collection of mutants, we have begun to clone and define the respective mutations in some of these strains. Direct selection of complementing cosmids offers a straight forward approach to identifying the underlying lesion associated with temperature-sensitive mutants in a number of organisms (for example, see Osherov et al, 2000). This is made simpler when the mutation being studied has been mapped on the genome. We have undertaken to complement temperature-sensitive mutants using cosmids mapped on the genome by the Neurospora genome program (Galagan et al. 2003).
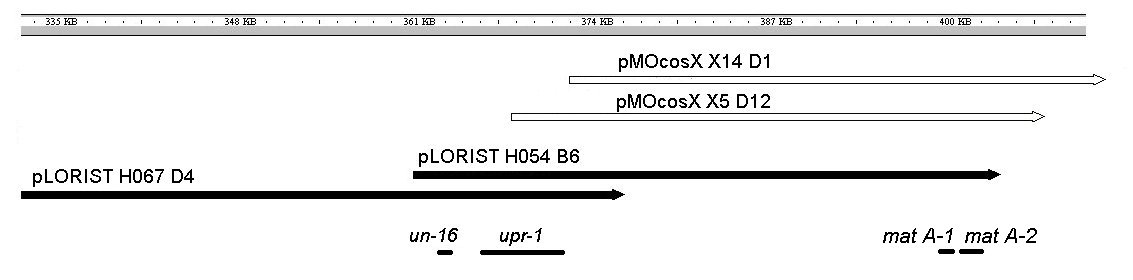
un-16 was known to be near mating type on linkage group I (Inoue and Ishikawa, 1970, Ishikawa and Perkins, 1983); therefore, we endeavored to walk to un-16 from the mat locus. Strain 4306 (un-16 A) was transformed using the protoplast protocol described by Vollmer and Yanofsky (1986) using cosmid DNA prepared by the Qiagen midi prep procedure. Selection was carried out by holding the plates at room temperature for several hours prior to incubating plates at 37C. Because the recipient strain does not have any secondary markers, we included a negative, no-DNA control. In eight transformation experiments, no revertants were seen on these controls. Cosmid pLorist6Xh 54B6 (Kelkar, et al. 2001) was one of the first chosen for the walk as it includes the mating type locus on its right end. This cosmid was found to complement un-16. Additional cosmids that contain genomic inserts overlapping the insert contained in cosmid 54B6 were used in transformation experiments to define the region of the genome containing the complementing gene (Table 1).
Table 1. Cosmids assayed for complementation of un-16
Cosmid |
total transformants |
Replicates |
pLorist6Xh 54B6 |
465 |
5 |
pLorist6Xh 67D4 |
131 |
6 |
pMOcosX* X5D12 |
1 |
2 |
pMOcosX X9B4# |
0 |
1 |
pMOcosX X14D1 |
0 |
1 |
pLorist6Xh 63H10* |
1 |
1 |
*Orbach and Sachs, 1991
#from assembly 3
The high efficiency of complementation by cosmids 67D4 and 54B6 encouraged us to look carefully at the 29 Kb overlap between these two cosmids (Figure 1). This area included five open reading frames, NCU01948.3, NCU01949.3, NCU01950.3, NCU01951.3 and NCU01952.3. Sakai et al. (2002) had previously complemented un-16 with NCU01949.3 as part of their investigation of upr-1. Our results, plus those of Sakai et al. lead to the conclusion that NCU01949.3 encodes the un-16 phenotype.

Figure 1. Overlapping cosmids complement un-16. Cosmids shown as solid arrows complement the un-16 mutation while those shown as open arrows do not.
Based upon its homology to other identified genes including one in Podospora, un-16 encodes the 40S ribosomal protein S9 (Dequard-Chablat, 1985). This protein is highly conserved among eukaryotes and archaea (Figure 2). The 40S ribosomal S9 protein is distantly related to the bacterial S4 ribosomal protein and has been found to function in maintaining the accuracy of protein synthesis (Vincent and Liebman, 1992). Sacchromyces cerevisiae is unusual in that it has two genes encoding the S9 proteins, S9-A and S9-B. Two mutations have been identified in yeast S9-B (SUP46-1 and SUP46-2) that result in increased misreading of mRNA by translating ribosomes (Vincent and Liebman, 1992). The respective positions of these mutations are listed in Figure 2.

Figure 2. Alignment of N. crassa un-16 with the 40S ribosomal S9 subunit. Nc, Neurospora crassa; Sc, Saccharomyces cerevisiae 40S ribosomal protein S9-B (accession number NP_009748); At, Arabidopsis thaliana 40S ribosomal protein S9 (accession number NP_197024); Hs, Homo sapiens 40S ribosomal protein S9 (accession number NP_001004); Mt, Methanosaeta thermophila PT, 40S ribosomal protein S4 (accession number YP_844026). Asterisks indicate the positions of the L34R and L103R mutations, respectively. The positions of the S. cerevisiae SUP46-1 (D94N) and SUP46-2 (L97W) are underlined in the Sc 40S rp S9-B sequence. The colors represent categories of amino acids: blue (acidic) - aspartate and glutamate; pink (basic) - arginine, histidine, and lysine; green (polar) - asparagine, cysteine, glutamine, serine, threonine, and tyrosine; red (non-polar) - alanine, glycine, isoleucine, leucine, methionine, phenylalanine, proline, tryptophan and valine.
To further characterize N. crassa un-16, DNA sequence analysis was used to define the respective mutations in two independent un-16 mutants, allele T42M38(t) in strain FGSC#4306 and allele T42M69 in strain FGSC#7558. Allele T42M38(t) of un-16 was found to contain a single nucleotide difference changing leucine codon 34 to an arginine codon, and the un-16 allele T42M69 contained a single nucleotide difference changing leucine codon 103 to an arginine codon (Figure 2). The L34R mutation is located in the N-terminal domain of the S4/S9 protein, whereas the L103R mutation is located within the S4 domain
N. crassa un-16 mutants were easily complemented by the wild-type allele suggesting that the un-16 mutations are recessive. However, because un-16 encodes a mutant ribosomal protein S9, it is possible that it could disrupt protein synthesis in heterokaryons with strains carrying a wild type un-16 gene. To address this issue, we attempted to make heterokaryons of un-16 and a strain with a wild type un-16 locus. Strains FGSC#4306 and FGSC#7558 were paired individually with strain FGSC#8964 (am33 arg-3 ad-3A) and with each other on minimal medium and incubated at 37C. In two separate replicates, both un-16 strains grew vigorously with FGSC#8964 at 37C while they did not grow at 37C when paired with each other or when alone. Strain FGSC#8964 did not grow alone on minimal medium.
The ease of use of un-16 as a selectable marker led us to clone the un-16 fragment by amplifying a DNA fragment containing the un-16 gene. Primers were selected to amplify a 1.5 Kb fragment and the fragment was cloned into the pSC-A cloning vector using topoisomerase I-mediated ligation followed by Cre-mediated recombination (Stratagene). DNA from eight of the resulting clones were prepared using a Wizard prep (Promega) and transformed into prepared un-16 protoplasts. Seven of the resulting clones complemented the un-16 lesion. One clone, pUN16-6, gave the best transformation and it is available through the FGSC.
Acknowledgements This work was supported by NSF grants 0235887 and 0235871.
References
Dequard-Chablat, M. 1985. Identification of the structural gene for the S9 ribosomal protein in the fungus Podospora anserina: a new protein involved in the control of translational accuracy. Mol. Gen. Genet. 200:343-5.
Galagan J.E., et al. 2003. The genome sequence of the filamentous fungus Neurospora crassa. Nature. 422:859-68.
Inoue, H., and T. Ishikawa. 1970. Macromolecule synthesis and germination of conidia in temperature-sensitive mutants of Neurospora crassa. Jpn. J. Genet. 45: 357-369.
Ishikawa, T. and D. D. Perkins. 1983. Additional irreparable temperature-sensitive mutants. Neurospora Newl. 30:9
Kelkar, H.S., J. Griffith, M.E. Case, S.F. Covert, R.D. Hall, C.H. Keith, J.S. Oliver, M.J. Orbach, M.S. Sachs, J.R. Wagner, M.J. Weise, J.K. Wunderlich, and J. Arnold. 2001. The Neurospora crassa genome: cosmid libraries sorted by chromosome. Genetics 157: 979-990.
Osherov N, J. Mathew and G.S. May. 2000. Polarity-defective mutants of Aspergillus nidulans. Fungal Genet. Biol. 31:181-8.
Orbach, M.J. and M.S. Sachs. 1991. The Orbach/Sachs cosmid library of N. crassa DNA sequences (pMOcosX). Fungal Genet. Newsl. 38: 97.
Perkins, D. D., A. Radford, and M. Sachs. 2001. The Neurospora compendium: chromosomal loci. Academic Press, San Diego.
Sakai, W., C. Ishii, and H. Inoue. 2002. The upr-1 gene encodes a catalytic subunit of the DNA polymerase ζ which is involved in damage-induced mutagenesis in Neurospora crassa. Mol Genet Genomics 267: 401-408
Vincent, A. and S.W. Liebman. 1992. The yeast omnipotent suppressor SUP46 encodes a ribosomal protein which is a functional and structural homolog of the Escherichia coli S4 ram protein. Genetics 132: 375-386.
Vollmer, S. J. and C. Yanofsky. 1986. Efficient cloning of genes of Neurospora crassa. Proc. Natl. Acad. Sci. USA. 83: 4869-4873.
Return to the FGN 54 Table of Contents