David D. Perkins - Hints and precautions for the care, feeding and breeding of
Neurospora
Some of these notes describe what may be established routines in older laboratories, but
are either unpublished or published in inaccessible places or scattered through the old
literature, and thus may not be known to those just beginning to use Neurospora. Others
may be practices unique to our lab. The attempt here is to supplement compilations of
methods such as Davis and de Serres (1970 Methods in Enzymology 17A:79-143) and the
older Stanford Neurospora Methods (1963 Neurospora Newsletter 4:21-25). Hints and
precautions regarding particular mutants or categories of mutants will also be found in
individual entries in Perkins et al. 1982 Microbiol. Rev. 46:426-570 (referred to below as
"Compendium"). Statements apply to N. crassa unless another species is
indicated. Neurospora Newsletter is abbreviated N.N. All temperatures are
degrees°C.
This document may be browsed or one can jump to any of the headings described below:
Crossing
Tips on handling ascospoes
General Laboratory Practices
Stockkeeping
Mutagenesis and enrichment
Tips for encouraging colonial growth
Solutions and media
Handling Heterokaryons
CROSSING
1. Temperature; glassware. Crosses are usually made at 25°C. Perithecia do not
develop, or do so poorly, above 30°C. We routinely make crosses on SC slants in
18 X 150 mm tubes, using foam or cotton plugs. Push-on metal or plastic caps should be
avoided with cross tubes because they result in more rapid water loss and are more
prone to contamination during the extended incubation period. Crosses are made on
petri dishes for specific purposes, as when shot asci are to be collected. But plates are
more prone to contamination and desiccation than tubes, and less compact for
incubation and storage.
2. Media and supplements. The synthetic crossing medium (SC) of Westergaard and
Mitchell is most widely used. The need to adjust pH of SC to 6.5 can be avoided by
substituting 0.7 g K2HP04 and 0.5 g KH2HP04 per liter for the monobasic salt in the
original formula (1963 N.N. 4:21-25). The formula in Davis and de Serres, p. 86,
incorporates this change. The trace elements used for Vogel's medium N are suitable
for SC at 0.1 ml per liter final volume; there is no need for a separate formula.
Russo, et al. (1985 N.N. 32:10-11) have modified Vogel's Medium N for use as a
crossing medium by reducing NH4NO3 tenfold. The modified Vogel's can be made up
as a 50X stock, whereas the Synthetic Cross formula of Westergaard and Mitchell allows
only 2x.
High ammonium or amino nitrogen inhibits crossing. Keep amino acid, purine or
pyrimidine supplements at a minimal level in crossing medium. If total amino acids do
not exceed 0.3 mg/ml there is usually no problem with fertility. Usually the parent with
the simplest requirements is preferred as female.
3. Use of per-1 to assure female parentage. Where the pedigree of a
mitochondrial genome or plasmid is critical, and assurance is desired that only one
parent is functioning as female, per-1 (type I) may be used. When
per-1 is present in one parent, all perithecia should be black and no white
perithecia should be present if the protoperithecial parent was per-1+, and
vice versa if the protoperithecial parent was per-1-.
4. False perithecia. In some single-mating-type strains, protoperithecia may enlarge and
become pigmented so as to resemble small perithecia. These "false perithecia" are
devoid of beaks, asci, and ascospores, and unfertilized strains exhibiting them remain
completely sterile. The strains can develop normal perithecia upon fertilization with the
opposite mating type. False perithecia are characteristic of single-mating-type cultures of
Neurospora tetrasperma and of the Kirbyville, Texas population of N.
discreta. False perithecia are also encountered sporadically in some N.
crassa genotypes - they are most commonly of mating type a. Some isolates of
fl a (fluffy) genotype have tended to make false perithecia.
The false perithecia can be a nuisance for mating-type testing, and a cause of alarm if
contamination is incorrectly suspected to be responsible. Ordinarily, continued complete
sterility and failure to develop even after long incubation distinguish false from true
perithecia, and there is no serious problem. False perithecia can be of serious concern,
however, in crosses where legitimate true perithecia are barren as a result of duplications
or of genes affecting meiosis and ascus development.
False perithecia are also an object of interest for those interested in sexual
differentiation. For example, inactive mutants of mating type a show development of
barren or false perithecia in abortive mating reactions with A or a
testers (Griffiths et al. 1978 Genetics 88:239-254; 1982 Can. J. Genet. Cytol.
24:167-176).
ASCOSPORES. OBTAINING PROGENY
5. Ripening of ascospores. Ascospores are unripe when first shot, even though fully
black. For good germination, ascospores should be aged 7-10 days at 25-30° (not
34°C) after shooting begins, before isolation.
6. Rehydration of ascospores. Ascospores from old cross tubes that are desiccated
should be rehydrated before heatshock to avoid poor germination (Strickland and
Perkins N.N. 20:34-35). This is most likely to be a problem with crosses in small tubes.
Rehydration can be accomplished conveniently by adding water to the cross tube or by
holding isolated ascospores overnight on moist fresh medium before heatshock. If
ascospores are isolated to slants prior to heatshock, fresh tubed medium gives better
germination that medium has partially dried down.
7. Ascospore viability and longevity. For ascospore maturation, some auxotrophs
require supplementation of crossing medium even though they are heterozygous,
recessive, and used as fertilizing parent. This is true of pan-2 (not
pan-1), spe-1, and some nic, cys and met genes
(see Compendium entries).
N. crassa ascospores may be stored in sterile water sealed in small vials
without appreciable loss of viability for at least a year at room temperature and 18
months at 4°C (B.R. Smith 1973 N.N. 20:34). Mature ascospores of N.
crassa also remain viable in ordinary cross tubes and can be germinated after many
months storage at 5°C. In contrast, germination of N. tetrasperma ascospores is
reported to decline after 19 days following simultaneous inoculation of A + a or after 11
days following inoculation of conidia from an (A + a) culture (Howe et al. 1966
Genetics 54:293-302).
When N. crassa perithecia become desiccated before all the asci within
them have been shot, ascospores remain viable in the unextruded asci and show high
germination months later when rehydrated and heatshocked. Intact linear asci may be
extruded singly after perithecia that have been dried in this way are placed in liquid.
Crosses made in 10 X 75 mm culture tubes often dry down before the perithecia have
emptied their contents (P. St. Lawrence), and are conveniently stored for future use.
8. Heatshock. For heatshock, a 60°C water bath is preferable to a hot-air oven.
(Greater latent heat, better heat transfer, more stable). Allow at least 30 minutes at
60°C to assure killing of vegetative cells with a water bath, double this for dry air.
Cover the bath to assure killing of conidia on tube walls.
Never remove ascospores from 60°C and then return to 60°C. Once
activated, ascospores are vulnerable to killing by heat.
9. Spontaneous germination. Some genotypes result in spontaneous ascospore
germination (for example, the unpigmented ascospores of per-1 Type I). A
significant fraction of ascospores carrying fl (fluffy) germinate spontaneously, at
least in some crosses. This becomes apparent when ascospores are aged after isolating
tetrads. Off-ratios may result if fl ascospores break dormancy before
heatshock and are therefore killed.
10. Suspension and dilution of ascospores. When suspending ascospores for dilution
series, add 0.1% Difco agar to increase viscosity and slow sedimentation; this
concentration remains liquid at room temperature. For over-layering, 0.6%, 0.8% or 1%
agar is used, and this solidifies.
11. Germination on surface of plates. It is convenient for some purposes to germinate
ascospores after they have been spread on the surface of prepoured 3% or 4% agar
plates containing minimal medium N or other other, appropriately supplemented,
medium. Percent germination can be determined easily and quickly. Most auxotrophs
and some morphological mutants can be distinguished from their wild counterparts, and
counted or isolated selectively. Ascospores are taken by loop from a cross tube to a
drop of water in the middle of the 3% agar plate; the desired number (about 200 per
plate) can be obtained by adding or removing spores while examining the drop at 40-60
X magnification under the dissecting microscope. Spores are distributed evenly over the
plate, using a sterile glass spreader. Do not spread to the extreme edge. Avoid
scratching the surface. If the germinating ascospres are to be scored but not picked,
ascospores may be suspended in 0.15% agar and spread in narrow parallel streaks with a
Pasteur pipette (Maling 1959 Stanford thesis).
Plates are heatshocked 30-40 min above the surface of a 60°C water bath with
lids off, and with the bottom of the dish just touching the water. The bath should be
covered. Alternatively, lids may be kept on if the plate being heat-shocked is placed on
a partially submerged water-filled container such as a petri dish, just above the surface of
the 60°C water bath. (Allow 60 min in a 60°C hot-air oven.)
In the absence of sorbose, inspection and isolation of germlings must be timed
carefully because fast-growing germinants will overgrow the plate. Rough estimates of
timing: 18 to 23 hours incubation at 20°C, 15 to 16 hours at 25°C, 5 to 7 hours
at 34°C, 11 to 12 hours at 39°C. (39°C is above the optimum.)
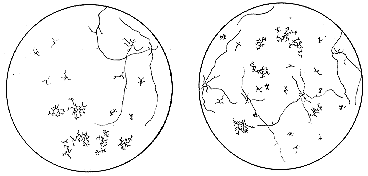
Figure 1. Camera lucida drawing of germinating ascospores showing the
segregation of two genes in a cross of col-4 x pyr-2. Spores were isolated on minimal
agar medium in petri dishes, heated at 60° for 30 minutes for activation, and
incubated at 25° for 18 hours. This nutritional mutant grows sufficiently on minimal
medium to permit identification of pyr-2 col-4 double mutants. One plate shows
segregations in two asci with spore pairs isolated in order. From top to bottom the two
asci show, respectively, genotypes pyr, pyr, col, col; and wild, pyr, col-pyr, col. The other
plate shows results from plating spores from the same cross at random. Genetic analyses
can be made directly using either procedure, or by transferring germinated spores to
appropriately supplemented culture tubes for further testing. Much larger numbers can
be observed conveniently using the random method. Reproduced with per-mission from
R.P. Wagner and H.K. Mitchell, 1955. Genetics and Metabolism, 2nd edition. J. Wiley
and Sons, Inc. N.Y.
12. Microscope and illumination. For most manipulations and observations
(ascospore isolations; inspection of spores, perithecia, etc.), an excellent general-purpose
microscope is the Stereo Zoom 7 with base, Bausch & Lomb Cat. No. BVIO70 with 10X
oculars (1984 price $1417). This provides 10X-70X magnification. Illumination from
above is best for isolating ascospores, with a white plate on the microscope stage to
maximize contrast of the viable black spores and distinguish them from spores that are
unripe or defective. Lighting from below using the frosted reflector is best for
determining the percent of defective, hyaline ascospores when scoring chromosome
aberrations. A balance of light from above and below is used for examining shot groups
of eight ascospores and classifying them according to numbers that are black or nonblack
(Perkins 1974 Genetics 77:459-489). Fixed- focus illuminators with snouts adapted for
convenient use with the Stereo Zoom 7 are available from American Optical (AO
*363H, $94) and B & L (Nicholas illuminator, B & L 31-33-05-28, $100). Lighting
intensity of both is just adequate for most purposes. Where more intense light is desired
at high magnification, a focusable illuminator may be preferred, such as AO 653H ($182)
or B & L 31-33-60-40 ($220).
13. Tools for manual isolation and transfer. Isolating and transfer needles are
readily formed from 70% platinum 30% iridium wire .020 inch diameter, obtainable from
Engelhard Industries, 700 Blair Rd., Cataret NJ 07008. (Obtain quote. Quantity
purchase is more economical because there is a minimum tooling charge for each order).
Platinum-iridium can be beaten and cut to make blades or needles are sharp, flat and
thin, as desired.
14. Use of glass spreader for ascospores. If ascospores are being spread on agar with
a glass spreader, use a separate spreader for each cross. Alcohol flaming is inadequate
to kill all adhering ascospores (Newmeyer et al. 1971 N.N. 18:13).
LAB PRACTICE, HOUSEKEEPING
15. Avoid drafts. Make transfers in a draft-free room. Don't walk breezily past
some- one transferring Neurospora. We carry out all routine operations (transfers,
isolations platings, etc.) at bench or table in an open lab, taking care to work near a
bunsen flame and to sterilize surfaces before and after use. Air circulation from air
conditioning is shut off while transfers are being made in the laboratory. Except for
manipulations involving massive transfers of conidia or opening profusely conidiating
plates, we have never found it necessary to work in a special enclosure or transfer room.
Where risk of conidial scatter is high, a nonventilated circulation-free tissue culture
enclosure has been used (Labconco 11000, 1984 price $1445). A small alcohol lamp can
be used within the hood. Fume hoods through which air is circulating should be
avoided; a regulation "biohazard hood" with its near-gale velocities would probably be a
catastrophe.
16. Decontamination of work space and glassware. Wipe bench surfaces before and
after use with 70% alcohol, or dilute phenol (Lysol), or dilute hypochlorite (household
bleach). Autoclave all contaminated glassware and media before discarding or sending
to dishwasher. Exceptions: Pipettes may be discarded into dilute hypochlorite.
17. Mite control. Infestation of cultures by mites can be a serious problem. Mites
can pass by or through plugs and closures, carrying conidia and other contaminants.
Don't leave cultures unnecessarily for long periods at room temperature, so as to risk
mite infestation. For the same reason, don't bring soil, plants, vegetables, fruit or other
possible sources of mites into the work area or into refrigerators where cultures are
stored. Large slants, which retain moisture for weeks or months before drying down, are
the most prone to become infested. My impression is that mites do not proliferate
effectively in cultures that are completely dried. This is based on very limited
experience.
If more than one different type of contaminant is found in a tube or plate, inspect for
mites (about the size and shape of protoperithecia; use 20-40X magnification). If mites
are found, autoclave or freeze cultures and accessories. Both eggs and adults are killed
after 24 hours at -18°C (Subden and Threlkeld 1966 N.N. 10:14). Clean infested
area scrupulously and treat equipment, benches, shelves, incubators, refrigerators and
surroundings with a miticide. Subden and Threlkeld reccommend Kelthane. Because
resistance may develop, Threlkeld (personal communication) suggests alternating
Kelthane and Malathion. We have, in the past, cautiously used Lindane applied to
incubators and benches in alcoholic solution (which leaves a residue), and have found it
highly effective. Lindane has since been listed as a carcinogen (see Merck Index), but
carcinogenicity data are said to be equivocal ("An Assessment of the Health Risks of
Seven Pesticides...". N.R.C. Board on Toxicology, Nat. Acad. Press, 1982).
Freezing kills ascospores but not conidia. A method for using carbon dioxide to rid
cultures of mites without killing ascospores has been described by Metzenberg (1985
N.N. 32:22).
18. Avoid saturated atmosphere in closed containers. Never enclose inoculated plates
or tubes in a small airtight container. Condensate in a saturated atmosphere creates a
film of moisture allowing mycelia to grow through plugs and out of petri dishes.
STOCKKEEPING
19. Avoid transcription errors. Read labels before and after transfer of stock
cultures.
20. Long-term preservation. Minimize vegetative transfer of "stem" stocks, to avoid
acquisition of mutations and rearrangements. Subculture from the primary stock to
make a working stock for fertilizing crosses, etc. The primary stock should promptly be
put into suspended animation, such as silica gel (Perkins 1977 N.N. 24:16-17; see also
N.N. 26:24-27), -20°C deep-freeze (Perkins 1973 N.N. 20:33), cryopreservation
(Jong, et al. 1979 N.N. 26:26), or lyophil (Barratt et al. 1959 Science 112:122-123).
The FGSC has a description of these techniques.
21. Silica gel. Make certain that silica gel tubes are securely sealed to exclude
moisture. Longevity requires sustained desiccation. Double-thickness parafilm is
effective for cotton-plugged tubes if the film is stretched to render it adherent and to
seal it tightly to the glass.
We decided to use plain 13 X 100 mm culture tubes with cotton plugs and parafilm
seals for silica gel stocks, in preference to screwcap tubes. With the latter, we could not
be confident that the seal was tight, and the constricted neck seemed to make pipetting
more difficult.
22. Heterokaryons for preserving difficult stocks. Aconidiate stocks, which show low
survival after freezing, can be homogenized and silica gelled, though this is considerably
more laborious than preparing conidial suspensions. If aconidiate strains are
hetcompatible with Oak Ridge and have a phenotype appropriate for forcing, they can
be preserved most easily as phenotypically wild-type conidiating heterokaryons in
combination with am1 ad-3B cyh-1 as a nonmating "helper" component
(Perkins 1984 N.N. 31:41-42). Such heterokaryons can also be used to advantage for
sheltering and preserving difficult genotypes. Because osmotic stocks are readily lost,
os stocks should be replicated and monitored or carried in heterokaryons.
Auxotrophs such as inl and others affecting membrane components have
short halflives in absence of supplement but show good survival if supplemented. I
doubt that their long- term survival on silica gel has been determined.
MUTAGENESIS, ENRICHMENT AND REPLICATION
23. Ultraviolet. For UV mutagenesis, commercially produced 20-watt germicidal
lamps that fit small fluorescent fixtures are convenient and inexpensive. A new lamp
may show violent fluctuations at first, becoming steady after a few hours use. Output
gradually declines as the glass becomes increasingly opaque with use. At each use, the
lamp should be allowed to warm up 10--15 min to get maximum 256 radiation.
Monitoring with a UV meter is essential where precision of dosage is important. Do not
expose eyes to UV light; wear protective goggles or ordinary glasses.
24. Chemicals. Chemical mutagens are potentially hazardous. Protocols for
inactivating mutagens and for decontaminating glassware may be found in chapters by L.
Ehrenberg and C.A. Wachtmeister 1977, pp. 401-418 in "Handbook of Mutagenicity Test
Procedures" (Kilbey et al., eds.). Elsevier/N. Holland Press.
25. Filtration-enrichment. For filtration-enrichment, the method of Applegate et al.,
employing sorbose liquid medium, is recommended (1978 N.N. 25:17; Yoder 1979 N.N.
26:2324). Avoid loss of surviving conidia in concluding steps by pelleting with dead
conidia as carrier (D.E.A. Catcheside; described in Yoder 1979).
26. Replication. Protocols for replication, applicable to strains with wild type or cot
morphology, are given by Nilheden et al. 1975 Hereditas 79:239-250 (see pp. 246-248);
Littlewood et al., 1972 J. Bact. 110:1017-1021. For examples of replication using
conidiating colonial strains see Schroeder 1970 MGG 107:291-304, DeLange et al. 1981
Genetics 97:247-259, Stadler et al. 1979 MGG 171:59-68, 1984 Mutat. Res.
127:39-47.
Plates for replication should be allowed to dry enough before use so that there is no
condensate or water-film on the surface.
COLONIAL GROWTH
27. Sorbose. Sorbose (used to induce colonial growth) caramelizes when autoclaved if
medium N or another medium containing NH4 is used. A
brown breakdown product is produced. This does not usually seem to affect
colonialization. But carmelization can be avoided by autoclaving a
sorbose-glucose-fructose 2OX solution separately from the medium and combining after
sterilization (D.E.A. Catcheside), or more simply by substituting Synthetic Cross medium
(which is NH4-free) for Medium N (T. Legerton). The
amount of sorbose that is needed depends on the medium. Formulas are given by Davis
and de Serres, p. 88.
28. Use of cot-1. cot-1, which grows colonially above 30°C, can be a
valuable adjunct to sorbose (or can be used without sorbose) for platings. (see e.g. D.G.
Catcheside 1966 Aust. J. Biol. Sci. 19:1039-1046; D.E.A. Catcheside 1970 ibid
23:855-865; Schroeder et al. 1980 pp. 55-62 in de Serres et al. (eds.), "Conference on
DNA Repair and Mutagenesis in Eukaryotes").
29. Spot-testing on plates. Spot-testing to score requirements or resistance is
accomplished routinely 25 spots per plate in 5 X 5 arrays, holding plates inverted to
avoid scatter, and cooling the needle in medium or in sterile water (R.H. Davis). Basic
spot- testing medium: 0.4 % sucrose, 0.8 % sorbose; incubation at 250 (Davis and de
Serres, p. 88).
SOLUTIONS, MEDIA
30. Stock solutions of supplements. Concentrated solutions of amino acids and other
growth factors can be kept conveniently for extended periods at 5°C unsterilized, for
addition to medium before autoclaving. Growth of microbial contaminants is prevented
by periodic addition of a drop of chloroform to the stock solution. Riboflavin is
bleached by visible light. Shield supplemented media from light, and keep stock solution
in amber bottle.
31. Inhibition by supplements. In designing media and supplementation, be alert to
inhibitions due to competition for transport. Some of these are documented in
Compendium entries for his, hom, ser-3, gua, arg, lys and leu. For
example, if his- or hom- ascospores are germinated on organic
complete medium containing casein hydrolysate, growth may not go beyond the
germination tube.
32. Color-coding. Color-coding of diagnostic media is convenient and less error-prone
than marking plugs, tubes, or plate lids. Grocery-store "U.S. Certified Food Color" (e.g.,
McCormick, Schilling or Co-op) is effective and is not inhibitory at concentrations giving
pale colors.
33. Effect of medium on conidiation. Conidiation of wild type varies depending on
substrate. Glycerol complete medium (no. 2 in Tatum et al. 1950 Am. J. Bot. 37:38-46)
was developed to maximize conidiation. Difco Neurospora Medium (a rich complete)
tends to inhibit conidiation. Factors affecting conidiation are considered by Turian 1964
Nature 202:1240 and by Davis and de Serres, p. 138.
HETEROKARYONS
34. Vegetative incompatibility. Wild populations of N. crassa are highly
polymorphic for het genes at numerous loci, so that heterokaryon-compatibility is rare.
Similarly, laboratory wild-types and marked stocks may be vegetatively incompatible with
one another because of heterogeneous background, and inbreeding often is required in
order to obtain het-compatible combinations. (See Mylyk 1975 Genetics
80:107-124; 83:275-284; Perkins 1975 ibid 80:87-105).
Strains of opposite mating types are het-incompatible with each other in N.
crassa. The vegetative-incompatibility of opposite mating types (but not of other
het genes) may be circumvented by introducing the recessive suppressor
tol into both partners (Newmeyer 1970 Can. J. Genet. Cytol. 12:914-926), or
by using a mutant mating-type allele. am33 has lost the
het-incompatibility function but not ability to mate; am1 has lost
both vegetative incompatibility and mating functions; these mutant mating types were
induced in OR background (Griffiths et al. 1978 Genetics 88:239-254). am1 has proved
useful as a helper component in heterokaryons (Perkins 1984 N.N. 31:41-42).
35. Counting nuclei. Nuclear counts in conidia (or hyphae) are readily made with a
fluorescent microscope after staining with DAPI and Hoechst 33258 (Raju 1982 N.N.
29:24- 26). Alternatively, conventional staining methods may be used (see e.g. Serna and
Stadler 1978 J Bact. 136:341-351), but they are more complicated and
time-consuming.
Return to the beginning of this document
Go to the FGSC methods page
Return to the FGSC Main page
