Using the ‘Buller phenomenon’ in experimental evolution studies of basidiomycetes
Duur K. Aanen,
Laboratory of genetics, Wageningen University, Wageningen, The Netherlands.
Fungal Genetics Reports 55:13-14
(Pdf)
In basidiomycetes, the monokaryons in a mating simultaneously exhibit two clearly distinct behaviors that can be considered as female and male roles, respectively: the acceptance of a nucleus, and the donation of a nucleus. However, since each monokaryon is a hermaphrodite, these two behaviors are difficult to distinguish. Here, I describe a simple method to select for the male role. A nucleus is serially passed through a non-evolving monokaryon, utilizing the ‘Buller phenomenon’. I describe some theoretical predictions that can be tested with this method.
![]()
Sexual reproduction in basidiomycetes starts with hyphal fusion between two monokaryons (haploid mycelia arising after spore germination; c.f. Casselton and Economou, 1985; Raper 1966). Subsequently, haploid nuclei are exchanged and reciprocally migrate throughout the existing mycelia to give rise to a dikaryon (Figure 1a).
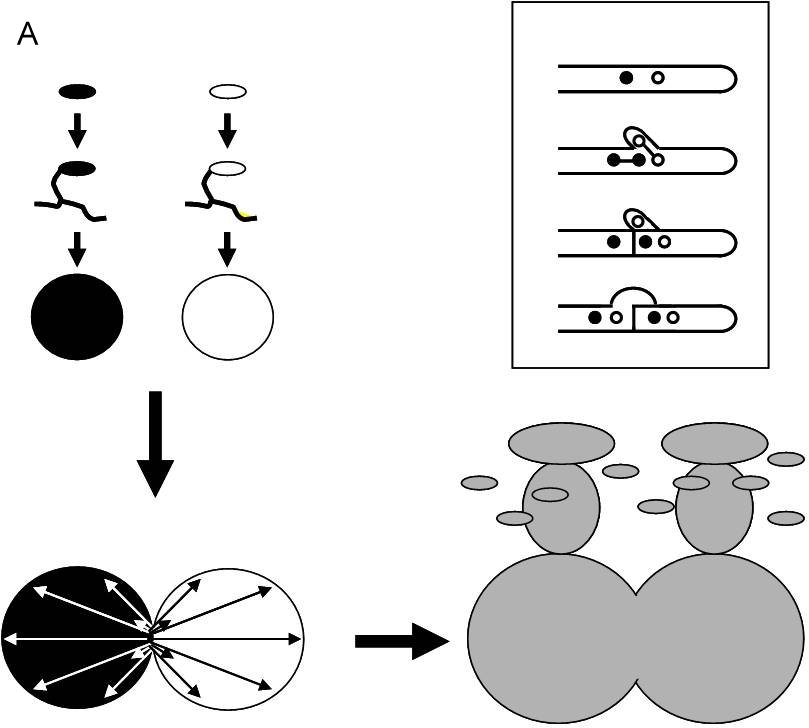
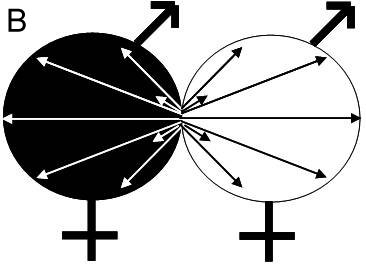
Figure 1. The standard life cycle of a heterothallic basidiomycete fungus. Sexual spores germinate (upper left) give rise to a haploid monokaryon. Two compatible monokaryons can fuse, upon which they reciprocally exchange nuclei, without cytoplasmic mixing (down left). This leads to the formation of the dikaryon (grey, right), all cells of which have two different nuclei, and which is a cytoplasmic mosaic. The dikaryon can produce mushrooms (schematically drawn on the grey dikaryon), the sexual fruiting bodies, where a short diploid stage is immediately followed by meiosis and sexual spore formation. The insert shows how the two nuclei in a dikaryotic cell are distributed over cells during cell division via the formation of clamp connections. b. The monokaryons exhibit two clearly distinct behaviors in a mating, accepting a nucleus, and donating a nucleus, which can be considered as female and male roles, respectively.
It has been shown that nuclear migration is faster than nuclear division, with estimated rates ranging from 0.5 to 3 mm per hour for Coprinus cinereus and Schizophyllum commune (Kües, 2000). This is up to 20 times faster than hyphal tip growth (Kües, 2000). The dikaryon can reproduce sexually via the production of fruiting bodies (basidiomata or mushrooms) bearing basidia. In these basidia, nuclear fusion occurs, followed by meiosis and the formation of basidiospores. Although the two monokaryons in a mating do not have specialized male and female organs, monokaryons exhibit two clearly distinct behaviors, the acceptance of a nucleus, and the donation of a nucleus (Aanen et al., 2004). The acceptance of a nucleus (with a contribution of the cytoplasm of that monokaryon to the newly established dikaryon) can be considered as a female role, while the donation of a nucleus (without a contribution of the cytoplasm of that monokaryon to the newly established dikaryon) can be considered as a male role and each monokaryon is a hermaphrodite (Figure 1b; Aanen et al., 2004). Therefore, mating between monokaryons is essentially asymmetrical. The dikaryon retains a male potential, as it can donate one of its nuclei after fusing with a compatible monokaryon to form a new dikaryon (Buller, 1931) by the ‘Buller phenomenon’ (Quintanilha, 1937).
Here I suggest making use of the Buller phenomenon (Quintanilha, 1937) in experimental-evolution studies of sexual selection, i.e. selection for mating success. For example, artificial selection for a fast nuclear migration rate can be done as follows (Figure 2): a nucleus X can be serially passed through a (non-evolving) monokaryon with nucleus Y, using the newly formed dikaryon with nuclei X and Y as the new donor of nucleus X.
To select for fast nuclear migration of X, the nuclei in the dikaryon, XY, that have migrated furthest (on the right in figure 2) will be selected for the next round. To prevent severe genetic bottlenecks, a broad front of the mycelium can be used at each transfer. During the experiment, little selection for mycelial growth takes place, because the non-evolving monokaryon Y ‘performs’ the mycelial growth. At the end of the experiment, the two nuclei X and Y can be separated by dedikaryotization of dikaryon XY via protoplasting (e.g. Sonnenberg and Wessels, 1987).
Subsequently, the migration rate of the evolved nucleus X can be measured (see Fischer, 2000, for possible methods) against a reference monokaryon and compared with the ancestral (non-evolved) nucleus X. One testable prediction is that the migration rate of X has increased during the experiment and it can also be tested whether the increased migration rate is associated with any trade-offs, for example, reduced mycelial growth rate of the monokaryon X and/or the resulting dikaryon.
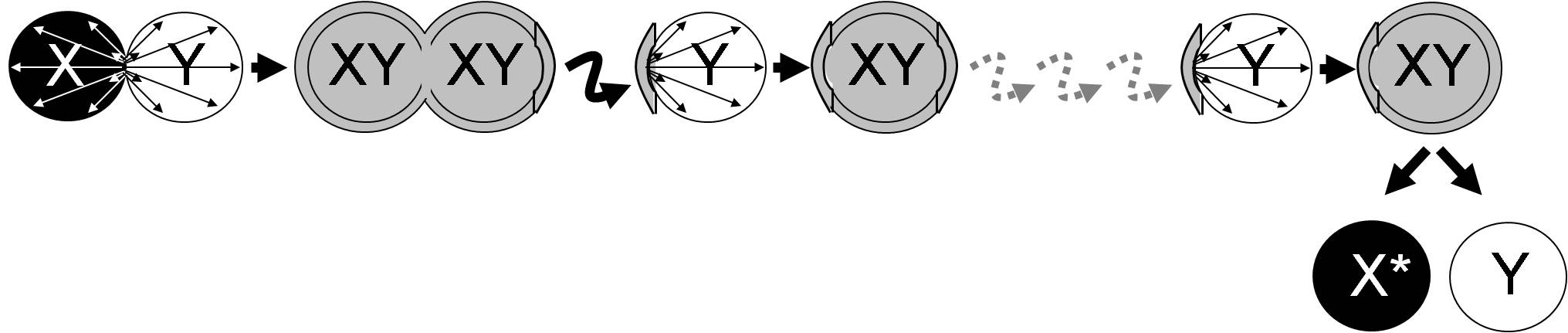
Figure 2. Experimental set-up for artificial selection for fast migration of nucleus X. Nucleus X is serially passed through monokaryon Y, which is not coevolving. This experimental set-up uses the ‘Buller phenomenon’, since the newly formed dikaryon XY fertilizes monokaryon Y. At the end of the experiment, the nuclear migration rate of the evolved X can be compared with that of the ancestral X.
References:
Aanen, D.K., Kuyper, Th.W., Debets, A.J.M. and R.F. Hoekstra 2004. The evolution of non-reciprocal nuclear exchange in mushrooms as a consequence of genomic conflict. Proc R Soc Lond B. 271, 1235–1241.
Buller, A.H.R., 1931. Researches on fungi, vol. IV. Longmans, Green and Co., London, United Kingdom.
Casselton, L. A. and A. Economou, 1985. Dikaryon formation. In Developmental biology of higher fungi (ed. D. Moore, L. A.Casselton, D. A. Wood & J. C. Frankland), pp. 213–229. Cambridge University Press.
Fischer, R. 1999. Nuclear movement in filamentous fungi. FEMS Microbiology Reviews 23 (1999) 39-68.
Kües, U. 2000. Life history and developmental processes in the basidiomycete Coprinus cinereus. Microbiol. and Molecular Biology Reviews 64, 316-353.
Quintanilha, A. 1937. Contribution à l’étude génétique du phénomène de Buller. C.R. Acad. Sci., Paris 205: 747.
Raper, J. R., 1966. Genetics of sexuality in higher fungi. New York: Ronald Press.
Sonnenberg, A.S.M. and J.G.H. Wessels. 1987. Heterokaryon formation in the basidiomycete Schizophyllum commune by electrofusion of protoplasts. Theor Appl Genet 74, 654-658.